Theory of the neuronal circuity of the brain and analytical thinking
ISBN 978-3-00-037458-6 ISBN 978-3-00-042153-2
Monograph of Dr. rer. nat. Andreas Heinrich Malczan
Part1.7 . The generation of the (magnocellular and primary) climbing fibre signal to a cortex cluster
Let us first summarise our insights so far:
- The cerebral cortex can be divided into cortex clusters.
- In each cortex cluster, a magnocellular activity neuron determines neuronal cluster activity by averaging over the other neurons in the cluster.
- The output signal of the activity neuron is - if there is sufficient and longer lasting neuronal cluster activity - converted into a neuronal system clock, which has a frequency of about 5 Hz.
- This signal is conducted from a GABAergic striosomal neuron - which is associated with the cluster - via an axon to the globus pallidus interna.
- The globus pallidus interna is a negative negation nucleus with external on-signal formation. It receives the excitatory input signal required for negation from the subthalamic nucleus. This single signal permanently excites a GABAergic output neuron.
- It is precisely this permanently excited output neuron that is strongly inhibited by the incoming output of the GABA-ergic striosome neuron when the system clock in the neuronal oscillating circuit just assumes the value 1. Otherwise, the strong inhibition does not occur.
- Therefore, the output of the associated neuron of the globus pallidus interna is the negated neuronal system clock.
- This output reaches the nucleus ruber as input. This is a positive negation nucleus. It forms its own excitatory input signal from the input it receives from the cortex, which contains, among other things, the original mean signals of all cluster neurons. This permanent signal is inhibited by the corresponding neuron of the globus pallidus. This negates the output of the globus pallidus. The result is the doubly negated neuronal system clock of the neuronal elementary oscillating circuit.
- This double-negated system clock from the associated striosome neuron is ultimately switched to the transmitter glutamate/aspartate in the nucleus olivaris inferior and reaches both the cerebellar nuclei and an associated Purkinje cell as a climbing fibre signal.
Thus the following theorem applies, which the author calls the Berlin theorem in honour of Germany's main state.
Each cortex cluster generates a magnocellular primary climbing fibre signal with the help of the striosome system of the basal ganglia, substantia nigra pars compacta, globus pallidus pars interna, nucleus ruber and nucleus olivaris inferior if cluster activity exceeds a threshold.
It seems necessary to give a separate name to the neuron in the nucleus ruber or in the nucleus olivaris, whose axon transports the primary magnocellular climbing fibre signal to the cerebellum.
Definition 1.19: Primary climbing fibre neuron
We refer to the neuron in the inferior olivary nucleus whose axon sends the primary magnocellular climbing fibre signal to the associated cerebellar cluster as the primary climbing fibre neuron of the inferior olivary nucleus.
We refer to the neuron of the nucleus ruber whose axon supplies input to the primary climbing fibre neuron in the nucleus olivaris inferior as the primary climbing fibre neuron of the nucleus ruber.
If the author refers to this magnocellular climbing fibre signal as primary, it can be assumed that there are obviously also secondary climbing fibre signals. These are derived from the primary climbing fibre signal by a special algorithm. This also clarifies the problem that there are very many Purkinje cells in each cerebellum cluster, to which only one primary climbing fibre signal is initially available. The first Purkinje cell or Purkinje group is assigned to the primary climbing fibre signal, the others receive a secondary climbing fibre signal. But more about that later.
It may be assumed that the climbing fibre signal serves to store the current signal combination in the cortex cluster. In anticipation of later explanations, we will refer to the signal stored in the Purkinje cell as the Purkinje cell's own signal. However, in order for a cortex signal to become the intrinsic signal of a Purkinje cell at all, it is necessary that the signals of the participating signal neurons of the cortex cluster find a path to the cerebellum. This is indeed the case.
Theorem1.16 : Projection of the signalling neurons of a cortex cluster into the cerebellum
The signalling neurons of each cortex cluster project excitatory into the cerebellum. Each signalling neuron first projects via the bridge nuclei to the cerebellar nuclei, where it has an excitatory effect on the output neurons of the cerebellar nuclei via collaterals. Furthermore, each signalling neuron projects into the cerebellum via a mossy fibre. The mossy fibres of all the signalling neurons in a cluster ultimately form a pair of parallel fibres via the mossy fibre projection into a cerebellum subarea, which in this theory is referred to as the associated cerebellum cluster. All Purkinje cells that tap this common parallel fibre population for input form a cerebellum cluster of Purkinje cells. All Purkinje cells in the cerebellum cluster are thus reached by the output of all the signalling neurons in the corresponding cortex cluster via the common pair of parallel fibres. Thus, each cortex cluster corresponds to an associated cerebellum cluster.
Note: Cerebellum clusters are already described in the English-language Wikipedia (as of June 2011). Newly added by the author of this monograph is the hypothesis that the mean neuron of a cortex cluster generates a primary climbing fibre signal for the associated cerebellum cluster exactly when the signal location in the cortex cluster is sufficiently active. Furthermore, the author postulates that the signal neurons of the cortex cluster project precisely into this cerebellum cluster. Thus, the author postulates the existence of a topological mapping of the cortex clusters to the cerebellum clusters. This topological mapping includes both the signalling neurons and the activity neurons of the cortex cluster.
As is well known, the output neurons of the cerebellar nuclei (with a few exceptions) are the only output station of the cerebellum. But the cerebellar nuclei not only output the cerebellum output, but also receive the input of the moss and climbing fibres via axon collaterals. It therefore seems highly appropriate to take a closer look at the structure and functioning of the cerebellar nuclei from a cytoarchitectonic perspective.
Susanne Kamphausen's dissertation on the "Functional architecture of the rat cerebellar nuclei" states the facts:
- There are (in the rat) two types of projection neurons in the cerebellar nuclei
- First, large exitatory projection neurons with fusiform or multipolar somata, 15 - 35 µm in size, 2 to 5 primary dendrites, spiny or smooth, transmitter glutamate, they project to motor centres of the brainstem, mesencephalon, thalamus and vestibular nuclei.
- Second, medium-sized GABAergic projection neurons, they innervate predominantly the inferior olive (93%), but also the cerebellar cortex or bridge, 5 - 20 µm, fusiform or multipolar somata.
- Thirdly, there are local inhibitory neurons 5 - 15 µm, transmitter GABA or/and glycine.
We read further in this dissertation, mutatis mutandis:
- Individual Purkinje cells form synapses with both glutamatergic and GABAergic projection neurons.
- The ratio of Purkinje cells to neurons of the cerebellar nuclei was estimated at 10:1.
(End of the non-verbatim reproduction from the above-mentioned dissertation)
How do we now imagine the circuitry of the cerebellar nuclei? Apparently, there are both positive and negative single-signal neurons in the cerebellar nuclei. Both derive their excitation from the input of the mossy fibres and climbing fibres. Both types of one-signal neurons are projection neurons, one glutamatergic and the other GABAergic. Both types of single-signal neurons are each strongly inhibited by the associated Purkinje cell by default. Only when a Purkinje cell recognises its own signal does this inhibition fail and the corresponding signals move to the target sites. The glutamatergic, i.e. excitatory signal moves to the thalamus (and from there to the cortex), the inhibitory signal to the olive.
It has already been shown in this paper that the subthalamic nucleus is a positive one-signal nucleus. Its single-signal neurons form their single signals by re-averaging the cortical average signals from layer V of the cortex.
It is astonishing that the principle of one-signal formation is also applied in the cerebellum nuclei. Even more interesting is that there are both positive, i.e. excitatory one-signals, and negative, i.e. inhibitory ones.
The input required for the formation of the single signal flows into the cerebellum nucleus via the collaterals of the mossy fibres on the one hand, and via the collaterals of the climbing fibres on the other. (Later it will be shown that each nuclear neuron is only contacted by one climbing fibre, while all moss fibres of a cluster supply each nuclear neuron with input).
Now, a pure one-signal, i.e. a neuronal continuous signal - regardless of whether it is inhibitory or excitatory - does not bring any gain in knowledge. But it is the prerequisite for generating an output through negation or inversion. In negation, the input signal is totally suppressed by the inhibitory input and thus leads to the negation of the input. With inversion, the inhibition by the input is imperfect and depends in its inhibition strength on the firing rate of the inhibiting input. With inversion, a residual signal remains after the inhibition of the input signal, the firing rate of which is approximately indirectly proportional to the original signal strength of the inhibiting input, if linearity is assumed.
So first of all, we postulate the existence of the positive and negative one-signal neurons in the cerebellar nuclei.
Theorem1.17 : Positive and negative one-signal neurons in the cerebellar nuclei
In the cerebellar nuclei, there are glutamatergic output neurons that act as positive one-signal neurons. Their on-signal excitation is generated by the excitatory input of the collaterals of the moss and climbing fibres. Their positive one-signal is inhibited by the associated Purkinje cells, producing an inversion of the Purkinje output. The positive input neurons of the cerebellar nuclei project excitatory mainly to the motor centres of the brainstem, the mesencephalon, the thalamus and the vestibular nuclei.
Similarly, GABAergic output neurons exist in the cerebellar nuclei and act as negative one-signal neurons. Their on-signal excitation is generated by the excitatory input from the collaterals of the moss and climbing fibres. Their negative on-signal is inhibited by the associated Purkinje cells, producing an inversion of the Purkinje output. The negative one-signal neurons of the cerebellar nuclei project inhibitory predominantly to the inferior olive (93%), but also to the cerebellar cortex or bridge. From a certain evolutionary developmental stage, they also project inhibitory into the nucleus ruber.
For every positive one-signal neuron in the cerebellar nuclei, there is a partner among the negative one-signal neurons that is inhibitively connected to the same group of Purkinje cells.
To shorten future texts, we will give the excitatory and the inhibitory output neurons of the cerebellar nuclei their own name.
Definition1.20 : Positive and negative nuclear neurons
We will refer to the glutamatergic output neurons of the cerebellar nuclei as positive nuclear neurons, and the GABAergic output neurons of the cerebellar nuclei as negative nuclear neurons.
It would be most gratifying if the neurological community could agree that (from a certain stage of development onwards) in addition to the inhibitory projection to the olive, there is also an inhibitory projection to the nucleus ruber (because, for example, the inhibitory projection axon divides and one collateral draws to the olive, the second to the nucleus ruber). This is urgently needed from a systems theory point of view, at the latest since the processing of the bridge nucleus output by the cerebellum. Therefore, this GABAergic projection from the dentate nucleus to the nucleus ruber will only occur in more highly developed mammals, while it is absent in the lower ones. Perhaps this is the reason for the contradictory data in the literature. The assumption found in this very literature that the nucleus ruber is a kind of "switching nucleus" that switches between the cerebrum and cerebellum when necessary could even be scientifically proven by an inhibitory projection from the nucleus dentatus to the nucleus ruber. It could also be proven what is actually being switched and for what purpose, and above all how this is done. That is why the undoubted existence of this inhibitory connection is so important.
Since it is generally accepted that several Purkinje cells each project onto an excitatory or inhibitory output neuron of the cerebellar nuclei, we want to give these Purkinje cells their own name.
Definition1.21 : Purkinje group
All Purkinje cells that project together with their inhibitory output both to the same positive nuclear neuron and also project together to the same negative nuclear neuron are called a Purkinje group.
A Purkinje group consists of at least one Purkinje cell. However, it can also comprise a larger number of Purkinje cells, for example three, eight or even thirteen Purkinje cells. The actual number of Purkinje cells in a Purkinje group depends on the evolutionary stage of development of the creature and the type of cerebellar nucleus. The different cerebellar nuclei of one and the same creature also represent different evolutionary developmental stages. Apparently, they developed through duplication, i.e. simple doubling. Since the newly formed (duplicated) partner nucleus did not yet have any tasks, it could develop further by modifying its cytoarchitecture and reach a higher level of system theory. The dentate nucleus has the highest level of development among the cerebellar nuclei.
The fact that a single climbing fibre can innervate several Purkinje cells is undisputed among neuronal experts. To do this, it splits up. Here, too, the literature varies between a single Purkinje cell, others give three, further sources eight to 13 Purkinje cells that are supplied by the same climbing fibre. The author therefore postulates that those Purkinje cells that send their output to the same output neurons of the cerebellar nucleus are also excited by the same climbing fibre. However, according to the above definition, these Purkinje cells form a Purkinje group. Therefore, the following theorem applies:
Theorem1.18 : Each Purkinje group is associated with exactly one climbing fibre and vice versa
Each climbing fibre always innervates only the Purkinje cells of exactly one Purkinje group of the cerebellum cluster as well as the associated excitatory and the associated inhibitory single-signal neuron of the cerebellum nucleus (nuclear neuron). Different Purkinje groups use different climbing fibres.
So there is a one-to-one mapping between the different climbing fibres and the Purkinje groups. There are (about) as many different climbing fibres as there are Purkinje groups.
The significance of combining several Purkinje cells into one Purkinje group will be clarified below.
As is well known, each Purkinje cell has a climbing fibre line. Even though three, eight or even up to thirteen Purkinje cells may now share a common climbing fibre and form a Purkinje group, it is initially unclear where the many climbing fibres are supposed to come from.
So far, it has only been shown that there is exactly one activity neuron per cortex cluster, from whose signal the striosome system of the basal ganglia generates exactly one primary climbing fibre signal by feedback and double negation. This single mean climbing fibre signal of each cortex cluster can now dock to exactly one and only one Purkinje group and supply it with input. We already want to give this special Purkinje group a special name for later classification.
Definition1.22 : Start group and primary magnocellular climbing fibre signal
The Purkinje groups of a cerebellum cluster are arranged one after the other along the moss fibre strand of this cerebellum cluster. We call the Purkinje group that is arranged first at the beginning of this mossy fibre strand the start group of the associated cerebellum cluster. We call the associated climbing fibre signal the primary magnocellular climbing fibre signal, and the corresponding axon the primary magnocellular climbing fibre.
All other Purkinje groups receive their climbing fibre signal via a different algorithm, which will be explained later. We will call them secondary (magnocellular) climbing fibre signals.
The evolutionary reason for the existence of primary and secondary climbing fibre signals lies in the progressive development of the cerebellum. Initially, there was only one Purkinje cell per cortex cluster. Later, a second, a third and a fourth were added. All these added Purkinje cells initially stored exactly the same signal. This made sense at first. In the neuronal death of a Purkinje cell, the system remained able to work. Therefore, the "surplus" Purkinje cells of a Purkinje group were the "iron reserve", so to speak. They formed a Purkinje group, which was also the starting group.
Later, other Purkinje groups were added, which were not actually necessary as reserve cells. Around this time, the Golgi cells may have gained in importance. Likewise, the olive and the nucleus ruber underwent a cytoarchitectonic expansion. This consisted of the use of the GABAergic projection from the cerebellum nuclei to generate a new, secondary climbing fibre signal.
This secondary climbing fibre signal was formed by a newly developed algorithm. It made it possible for the second Purkinje group to store a different signal than the first group, which was also the start group. And later, the newly added third group could store another, a third signal. So in the modern cerebellum, each Purkinje group stores a new signal that is completely different from the previous signals stored.
The actual system-theoretical task of a Purkinje group consists in learning and recognising a signal that flows as input from the cortex via the bridge nuclei and the mossy fibres into the parallel fibres and is learned or recognised.
There are two variants here:
- The signal was recognised. Then an excitatory projection was made from the cerebellum nucleus to the thalamus and an inhibitory projection to the olive and possibly also to the nucleus ruber.
- The signal was not detected. Then there was (almost) no output from the cerebellar nucleus.
According to the author, the inhibitory projection of the cerebellum nucleus neuron to the olive causes the suppression of the climbing fibre signal. This is because this neuron only fires when the Purkinje group recognises its stored intrinsic signal. At this precise moment, the output of the negative nucleus neuron suppresses the existing climbing fibre signal, as long as the intrinsic signal is present at the Purkinje group.
Since the climbing fibre signal represents the neuronal "write command", the climbing fibre signal is now suppressed and thus prevented from imprinting another, further (and free) Purkinje group. Only in this way could the different Purkinje groups learn different signals. This is because a signal that had already been learned led immediately to the inhibition of the climbing fibre signal whenever it was applied to the parallel fibres and recognised by the Purkinje cell. This prevented further "learning processes" with the already learned signal.
However, the inhibitory output of the negative nuclear neuron to the olive served not only to inhibit the primary climbing fibre signal. The absence of this inhibition also served to generate the secondary climbing fibre signal.
For the generation of the secondary climbing fibre signals, evolution developed two procedures (according to the author of this monograph).
The evolutionarily older method consisted of a precisely central climbing fibre neuron per cerebellum cluster in the nucleus olivaris. From this, all secondary climbing fibre signals were derived by central distribution. Each Purkinje group thus received exactly the output of the central climbing fibre neuron via an axon collateral. Each Purkinje group sent the inhibitory axon of the negative nuclear neuron from the cerebellar nucleus precisely to this central climbing fibre neuron and inhibited its excitation whenever this group recognised its own signal. Thus, this central climbing fibre neuron could only fire when it was strongly excited by the mean neuron of the cortex cluster and none of the already imprinted Purkinje groups recognised this signal as their own signal. This ensured that only unknown signals were stored as new signals.
Sketch1.20 : Central distribution of the climbing fibre signal (evolutionarily oldest variant)
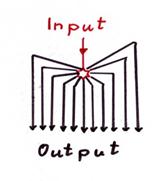
Note: In the above sketch, only the distribution of the output of the central climbing fibre neuron of the olive is shown, all inhibitory conductions from the negative nuclear neurons to exactly the single central neuron are missing.
In the case of central distribution of the climbing fibre signal, the primary climbing fibre signal of the cortex cluster excites exactly one central climbing fibre neuron of the olive, from which all Purkinje groups obtain their climbing fibre axon. Thus, all climbing fibre axons would initially always be synchronously excited. But the negative core neuron of each Purkinje group inhibits this central climbing fibre neuron exactly when it is active itself, so that all climbing fibre axons are signalless at the same time.
The second variant of generating the secondary climbing fibre signals from the primary climbing fibre signal was the sequential distribution. It emerged from the central distribution when the number of Purkinje groups became too large to be dominated by a single central neuron of the olive. Now the climbing fibre signal was passed on in a neuron chain, at the beginning of which was the primary climbing fibre neuron. Therefore, in the case of recognition, each Purkinje group inhibited the climbing fibre neuron of the neighbouring Purkinje group with its negative core neuron, so that its imprinting was impossible if the predecessor group had recognised the adjacent signal.
Sketch1.21 : Sequential distribution of the primary climbing fibre signal (mid-evolutionary stage).
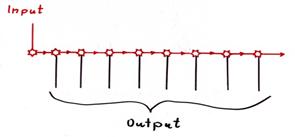
Note: In the above sketch, only the sequential distribution of the output of the primary climbing fibre neuron of the olive is shown, all inhibitory conductions from the negative nucleus neurons to the distribution neurons are missing. The output is not passed on centrally here, but with the help of excitatory interneurons or via axon collaterals in a chain.
The output of the negative nuclear neuron of the start group among the Purkinje groups inhibits the primary climbing fibre neuron in the inferior olivary nucleus.
The output of this primary climbing fibre neuron of the olive occupies a free neuron in the olive and excites it with its output. This occupied neuron provides the climbing fibre signal for the neighbouring Purkinje group of the start group.
The output of the kth negative nuclear neuron of the kth Purkinje group inhibits the climbing fibre neuron of this Purkinje group. This climbing fibre neuron is the kth climbing fibre neuron of the corresponding cerebellum cluster. The output of this kth climbing fibre neuron of the olive is the input for the (k+1)th climbing fibre neuron of the olive, which supplies the neighbouring group of the kth Purkinje group, i.e. the (k+1)th Purkinje group, with climbing fibre input.
This process of forming the climbing fibre input for the neighbouring Purkinje group continues until all Purkinje groups of the cerebellum cluster are used up and no new ones can form. Similarly, this recursive process occurs when the corresponding neurons in the olive are depleted.
Here, too, all climbing fibre axons are initially synchronously excited until a Purkinje group recognises its own signal and its negative nuclear neuron has an inhibitory effect on the feeding climbing fibre. All subsequent climbing fibres thus also become signalless.
Sketch1.22 : Generation of the secondary climbing fibre signals by sequential distribution in the olive and recurrent inhibition of the distribution neuron by the negative nuclear neuron.
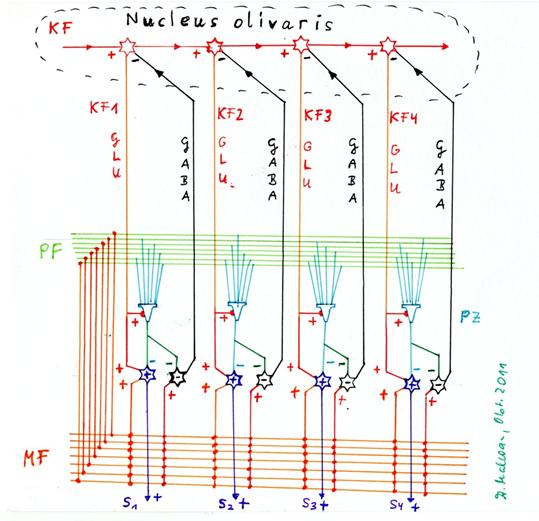
The above sketch shows four Purkinje cells, the positive (+) and negative (-) nuclear neuron for each, the mossy fibres, the parallel fibres, the primary climbing fibre axon KF, the four distribution neurons for the climbing fibre signal in the olivary nucleus and the inhibitory projection of each negative nuclear neuron to the corresponding distribution neuron for the climbing fibre signal in the olivary nucleus. Star cells, basket cells and Golgi cells are not shown, as only the generation of the secondary climbing fibre signals is involved here.
Now, inhibition of the climbing fibre signal in the case of signal recognition is an important necessary prerequisite, but it is not yet a guarantee that all Purkinje groups of a cerebellum cluster will not be imprinted with the same imprinting signal. Theoretically, it would be quite conceivable that all Purkinje groups, indeed all Purkinje cells, could be imprinted with the same signal in one ride.
However, this is precisely what must be prevented in reality. After all, it is sufficient if all cells within a Purkinje group store the same signal and thus there is a fail-safe against the neuronal death of individual Purkinje cells. Imprinting all Purkinje groups with the same signal would be a tremendous waste of resources.
Following these explanations, the focus must therefore be on the Golgi cells of the cerebellum. Until the publication of this monograph, the neuronal literature lacked a (plausible) system-theoretical justification for the existence of Golgi cells of the cerebellum. A certain contrast enhancement, which is suspected, is undisputed. However, more findings are not yet available.
Therefore, we first collect the facts on the subject of Golgi cells. In the textbook "Anatomy Volume 4" by Graumann/Sasse we read literally on page 279:
(start of quote:)
"Golgi cells are large inhibitory interneurons. They are sparsely distributed in the stratum granulosum but have a dendritic tree that branches widely in all directions of space. They control the activity of the granule cells."
(end of quote)
It can therefore be assumed that there are significantly fewer Golgi cells than Purkinje cells.
If, on the one hand, there are significantly fewer Golgi cells than Purkinje cells, and on the other hand, the Purkinje cells form groups that are controlled by the same climbing fibre and whose output converges on a positive and a negative single-signal neuron of the cerebellum nucleus, there is only one reasonable explanation: each Purkinje group forms a unit with exactly one Golgi cell. Then there should be about as many different climbing fibres as there are Golgi cells. Likewise, the number of Golgi cells should roughly correspond to the number of positive or negative single-signal neurons in the cerebellar nuclei.
We already assume that these criteria apply approximately and formulate this in a theorem.
Theorem1.21 : Coupling of the Purkinje groups with a Golgi cell
Each Purkinje group is terminated at its end by a Golgi cell.
The starting group of the cerebellum cluster ends with a Golgi cell, which is followed by the next Purkinje group, which in turn ends with a Golgi cell. This continues until the end of the chain of Purkinje groups.
Thus, there is one Golgi cell between each two Purkinje groups. After this elementary naming of the facts, we must derive the system-theoretical function of the Golgi cells.
It is known that the Golgi cells form complex synapses involving the moss fibres on the one hand and the granule cells on the other. It is assumed here that the Golgi cells generally have an inhibitory effect because they use the transmitter GABA. The most common assumption in the literature is that the Golgi cell inhibits the transmission of the mossy fibre input to the granule cells. In this case, the axon of the Golgi cell pushes itself between the axon of the moss fibre and the dendrites of the granule cell. This creates a complex synapse with three partners involved.
On the other hand, one also finds representations in the literature in which the Golgi cells form inhibitory synapses with both the moss fibres and inhibitory synapses with the granule cells, but these are spatially separated from each other.
The author decides to prefer the latter variant for system-theoretical reasons. In this case, the Golgi cell on the one hand inhibits the transmission of the excitation along the mossy fibre, which becomes signalless from the inhibitory synapse on. On the other hand, the Golgi cell also inhibits the accessible ascending axons of the granule cells, so that these become signalless even before they reach their branching into the so-called parallel fibres. In this way, a double inhibition of transmission is effected: signal transmission is suppressed both on the moss fibre axons and on the granule cell axons.
Here it is worth recalling literature source 42, which is listed in the list of resources used. In an excellent internet script by Prof. John K. Harting of the University of Wisconsin Medical School under the title "The Global Cerebellum '97", one finds the illustration of the coupling of moss fibres, granule cells and Golgi cells illustrated by David P. Van Lieshout. This excellent illustration may be used here as a picture quotation, which is quite permissible in scientific papers. It can be clearly seen in this illustration that they do not necessarily have to be "complex synapses". The inhibitory synaptic contact of the Golgi cell to the moss fibre can also be spatially separated from the excitatory synaptic contact of the moss fibre to the granule cell. In this case, the transmission of the moss fibre signals along the moss fibre would always be interrupted when the Golgi cell inhibits the moss fibre.
Sketch 1.23: Picture citation: Golgi cells inhibit moss fibres - source see (41):
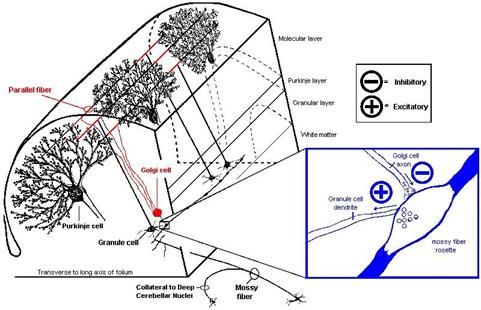
(end of the picture quotation)
We put this into a new theorem.
Theorem1.22 : Double conduction inhibition by the Golgi cells
Each Purkinje group is terminated at its end with a Golgi cell. This Golgi cell causes a double conduction inhibition in the cerebellum cluster.
On the one hand, when active, this Golgi cell inhibits all active moss fibre signals from propagating along these moss fibres to the neighbouring Purkinje groups, so that they cannot receive any parallel fibre input via granule cells that tap the moss fibres in question behind the Golgi cell.
On the other hand, this Golgi cell inhibits a large part of the granule cells in its immediate vicinity, so that their parallel fibre axons become signalless.
Of course, the question arises as to what purpose this double forwarding inhibition serves. It will be seen that this effectively prevents multiple imprinting of different, neighbouring Purkinje groups with the same imprinting signal. The neighbouring Purkinje group cannot be imprinted with the same signal, as this no longer reaches it due to the double forwarding inhibition. However, the prerequisite is that the corresponding Golgi cell is activated at the right time so that the double inhibition of forwarding occurs.
This is necessary if an unprinted Purkinje cell is to be imprinted by means of a climbing fibre signal. In this case, the double forwarding inhibition prevents the neighbouring Purkinje groups from receiving the moss fibre signal. Thus, it becomes impossible to imprint many neighbouring Purkinje groups with the same signal in "one ride". How is this realised in the cerebellum?
The argument that an imprinted Purkinje cell prevents the imprinting of further cells with the same signal by the fact that its associated output neuron in the cerebellar nucleus effectively suppresses the climbing fibre signal in the olive does not count here. This is because the question also refers to a point in time when the current imprinting signal has not yet occupied (i.e. imprinted) a Purkinje cell.
Here we recall the quotation from the "Anatomy" by Graumann/Sasse on page 280, which may be reproduced here once again:
"Climbing fibres contact dendrites of Golgi cells. These inhibit the granule cells and thus interrupt the transmission of the moss fibre input."
Thus, if a Purkinje group receives the strongly excitatory climbing fibre signal, this climbing fibre signal not only excites the Purkinje cells of the group, but also the Golgi cell present at the end of the group. This interrupts the transmission of the mossy fibre input to the other neighbouring Purkinje groups of the cerebellum cluster.
Without moss fibre input, however, the parallel fibres receive no signals, so that imprinting by a climbing fibre signal is no longer possible. This is because both long-term depression and long-term potentiation require input via the parallel fibres in addition to the tetanic (higher frequency) input via the climbing fibre axon. This applies both to the long-term depression of the synapses between parallel fibres and Purkinje cells and to the synapses between parallel fibres and stellate cells or basket cells. Therefore, especially because of their dendritic contact to the climbing fibres, the Golgi cells are excellently suited to choke off the input via the moss fibres during the imprinting process and thus prevent the imprinting of neighbouring Purkinje groups.
If we think of the Purkinje groups as being arranged along the parallel fibres and assume that (initially) there may also be a Golgi cell between every two Purkinje groups, it becomes understandable why a climbing fibre signal can only imprint the first free Purkinje group of the row. The Golgi cell following the first free Purkinje group interrupts the signal flow between moss fibres and granule cells due to the strong excitation it receives because of the climbing fibre contact. Therefore, the remaining Purkinje groups, stellate cells and basket cells that follow the first (free) Purkinje group cannot receive any excitation via the parallel fibres. This is true even before the first free Purkinje group has been imprinted by this climbing fibre signal. For the development of long-term potentiation (LTP) as well as long-term depression (LTD), however, not only the tetanic excitation by the incoming climbing fibre signal is necessary, but the excitation of the cells via the parallel fibre signals is equally important. However, these are completely "stalled" by the first Golgi cell that follows the first free Purkinje cell. Therefore, an incoming climbing fibre signal can only ever imprint the first free Purkinje group, while the rest receive (almost) no parallel fibre input at all. This is why the Golgi cells in the cerebellum have an important function. However, this task is not their only one, as will be shown later. It will also have to be clarified why the climbing fibres also contact the basket cells and stellate cells in addition to the Purkinje cells and Golgi cells.
The above statements apply (initially) under the restrictive condition that the length of the parallel fibres and the spatial extension of a Purkinje group in the direction of the parallel fibres do not differ significantly from each other and that the granule cells, from whose axons the parallel fibres ultimately arise, are predominantly located at the beginning of a Purkinje group. It is also assumed that there is signal attenuation in the propagation of the signals along the parallel fibres, and that the speed of propagation of the action potentials along the parallel fibres is significantly lower than along the mossy fibres. Later we will see that an increasing length of the parallel fibres across the boundary of a Purkinje group to the neighbouring groups brings additional imprinting possibilities. (See part 2.13. "The cerebellar reverberation" in this monograph).
The function of the stellate cells and granule cells present in the cerebellum will be explained in the following. But before that, we must look at the phenomenon of the imprinting of the Purkinje cells. According to the author's theory, the imprinting of Purkinje cells is mainly caused by long-term depression and long-term potentiation. This was suspected a long time ago. This monograph will attempt to provide exact proof of this.
In computer RAM memories, the content of a RAM cell is stored by a write command. If such a memory cell receives a write command, it permanently stores exactly the data that is on the data lines at that moment as binary values. The stored data can be read out again later with a read command. They are only lost when the power supply to the computer is switched off.
The author claims that the storage of data in Purkinje cells is done analogously via a write command. He even assumes that the write command is the climbing fibre signal, about which many assumptions have been made so far.
If this were true, there would be another commonality between the computer and the brain besides the neuronal system clock: the write command to store.
The Purkinje cells would then be classified as neuronal RAM memory.
The work "The Brain" by Richard F. Thompson from Spektrum Akademischer Verlag was the author's first neurological reference book, which he received as a gift from his work colleagues on the occasion of his birthday - in the form of a voucher.
In this excellent book, F. Thompson describes the discovery of long-term potentisation on page 104. The reader is offered a verbatim quotation from it:
(beginning of quote - page 104)
"When the Norwegian scientist T. Lømo and his British colleague Tim Bliss studied how the hippocampus responds synaptically to electrical stimulation of its afferent pathways in Per Anderson's laboratory in Oslo in 1970, they made a discovery: when they stimulated an afferent pathway at a high frequency for a short time (for example, at 100 hertz for one second, i.e. 100 stimuli per second), the hippocampal synaptic response to single test current surges at the same pathway increased dramatically, and this strength of response was maintained over the entire test period (Figure 4.14). The investigators called this phenomenon long-term potentiation (LTP)."
"The remarkable thing about this potentiation was that it lasted so long after such a brief irritation."
(end of quote)
It is permitted to also use the above-mentioned illustration here as a "picture quotation" so that the process of long-term potentiation is illustrated pictorially.
Sketch 1.24: LPT and LTP in the hippocampus - (Image citation - page 105 - Figure 4.14):
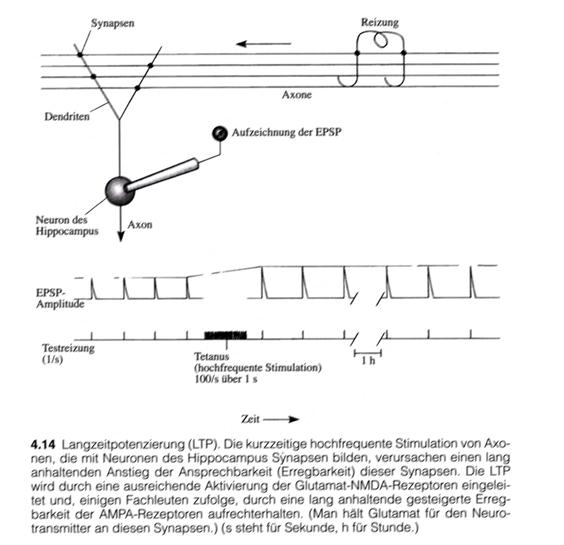
(End of the image quotation for long-term potentiation)
The drawing shows a cell of the hippocampus that can be excited via axons. The excitation is transmitted from the axons via the synapses to the dendrites of the hippocampal neuron and leads to an increase in membrane voltage. The latter is measured electronically.
If the feeding axons are stimulated by external application of suitable electrical signals, as shown in the figure, the change in membrane potential can be recorded.
A short-term, high-frequency excitation - known in neurology as tetanic excitation - leads to the development of long-term potentiation in the hippocampal neuron. Long-term potentiation significantly increases excitability over a longer period of time. This effect lasts for a long time. LTP and LTD have long been regarded as mechanisms for the development of long-term memory. Until now, the only thing missing was a concrete neuronal circuit that could be demonstrated in the real brain. The author attempts to present this here.
Source of the quotations: "The Brain" by Richard F. Thompson from Spektrum Akademischer Verlag, 2001
Long-term potentiation (LTP) is only one variant, a second one is long-term depression (LTD). In this second variant, the same cause - a tetanic (higher frequency) stimulation and simultaneous presynaptic excitation causes a strong and permanent reduction in synaptic coupling.
After this text and image quotation, we take the precaution of gathering all the facts about the cytoarchitecture of the cerebellum that have not yet been mentioned but are now known worldwide, in order to explain the imprinting algorithm for the Purkinje cells.
First of all, some peculiarities of the climbing fibre projection will be discussed, which will later facilitate the understanding of how the imprinting of the Purkinje cells works. In volume 4 of "Anatomy" by Graumann/Sasse entitled "Sensory Systems-Skin-CNS-Peripheral Conduction Pathways" by Schattauer-Verlag we read on page 208:
(start of quote:)
"Overall, climbing fibre excitation is modulated by the same mechanisms as moss fibre excitation:
- Climbing fibres contact the stellate cells with their axon collaterals; these inhibit the Purkinje cells in the sense of forward inhibition.
- Climbing fibres activate basket cells via axon collaterals, which inhibit parallel-connected Purkinje cells and thus cause lateral inhibition.
- Climbing fibres contact dendrites of Golgi cells. These inhibit the granule cells and thus interrupt the transmission of the moss fibre input. This is the only place in the cerebellar cortex where the climbing fibre system can influence the moss fibre system. The inhibition that occurs here is called heterosynaptic inhibition because it occurs between two different systems."
(end of quote)
The basket cells here are also quoted from the textbook "Anatomy Volume 4" by Graumann/Sasse. On page 279 it says:
(start of quote:)
"Basket cells are medium-sized inhibitory interneurons. They are located in the stratum moleculare, near the perykarya of PURKINJE cells. Their dendritic tree extends into the stratum moleculare, and their axon spins around the perykaryon and axon hillock of eight to ten neighbouring PURKINJE cells with numerous fine branches in a basket-like manner. Basket cells are responsible for lateral inhibition of PURKINJE cells."
(end of quote)
At this point we would like to thank the editors, Prof. Walter Graumann from Tübingen and Prof. Dieter Sasse from Basel, as well as all the co-authors involved, for this extremely successful technical book. Its special quality lies in the clarity of the explanations, so that ultimately even a mathematician could develop detailed ideas about the inner architecture of the neurological subsystems.
If the basket cells are medium-sized interneurons located near the cell bodies of the Pur-kinje cells, one could conclude that each Purkinje cell has its own basket cell. Otherwise, there would be quite a few Purkinje cells with no basket cells near them. Such an irregular distribution would certainly have been mentioned in the literature. Therefore, we postulate that each Purkinje cell has its own basket cell. We will refer to this basket cell as the specific basket cell of the Purkinje cell. If several basket cells belong to a Purkinje cell in the vicinity and interact with it, we will mentally combine them into a single, abstract basket cell, so that this one (imaginary) basket cell performs the desired work as a substitute.
Definition1.23 : Specific basket cell of a Purkinje group
A basket cell whose cell body is in the vicinity of a Purkinje cell, such that both the Purkinje cell and the basket cell contact a certain selection of parallel fibres in common, is called a specific basket cell of the Purkinje group.
Theorem1.23 : Basket cell theorem
In each Purkinje group, each Purkinje cell has a specific basket cell in its immediate vicinity. This basket cell receives excitatory input from the accessible parallel fibres and also receives excitatory climbing fibre input. This basket cell itself inhibits all Purkinje cells of the Purkinje group to which it belongs when excited.
The theorem does not assume that a basket cell cannot have an inhibitory effect on Purkinje cells of neighbouring Purkinje groups. However, the inhibition of all Purkinje cells of the same group is much more important for learning ability and is therefore given special credit. It will be shown later that each basket cell inhibits all Purkinje cells in a certain spatial area, so that a kind of receptive inhibition field is created. Here it would be necessary to show that the axon branches radially in all directions and that each partial axon inhibits, for example, eight to ten Purkinje cells. If the cerebellum layer is cut open along a plane, only the axon branching parallel to the plane of the cut can be seen, while the other branchings do not run close to the surface and are thus hidden by the rest of the material. So this still needs to be checked.
To prove that the cerebellum is a storehouse of complex signals that is sequentially organised, we still need some now well-established facts about the effect of Purkinje cells on the interneurons of the cerebellum.
The Purkinje cells here are also quoted from the textbook "Anatomy Volume 4" by Graumann/Sasse. On page 281 it says:
(start of quote:)
"Additional modulation occurs via the efferent neurons of the cerebellar cortex, the Purkinje cells. These send axon collaterals to all interneurons of the cerebellar cortex. The resulting inhibition of inhibitory neurons leads to a reversal of inhibition, disinhibition."
(end of quote)
From the above quotation, an essential implication for the Golgi cells can be derived, which is extremely significant for the system-theoretical functioning of the cerebellum:
The Golgi cells are inhibited by axon collaterals of the Purkinje cells.
This fact is extremely important for the control of the forwarding inhibition along the moss fibres, especially in the case of non-recognition and the recognition of a signal by a Purkinje cell. The forwarding inhibition in the case of signal detection has a functional counterpart: the suppression of the forwarding inhibition in the case of non-detection. Since a non-recognising Purkinje cell is strongly excited, it inhibits the Golgi cell with its excitation so that it is inactive. As a result, the moss fibre signals from the Golgi cell cannot be inhibited from propagating further along the moss fibres to the neighbouring groups. The forwarding inhibition can therefore be switched on by an active climbing fibre signal and switched off by the active Purkinje cell.
The fact that basket cells and stellate cells can also be inhibited by the collateral axons of Purkinje cells will prove useful later.
Since, in the author's view, the smallest organisational unit of the cerebellum is the Purkinje group, each terminated at its end by a Golgi cell, we assign the inhibitory axon collaterals of the Purkinje groups to exactly this one Golgi cell. Thus, according to the author, each Golgi cell is inhibited by the Purkinje cells of the Purkinje group to which it belongs. The meaning of this inhibitory connection remains to be seen. However, we put this connection into a separate theorem.
Theorem1.24 : Inhibition of the Golgi cell by the Purkinje cells of the associated Purkinje group
The Golgi cell of a Purkinje group is inhibited by the Purkinje cells of that group when these Purkinje cells are active.
At the end of the theoretical presentations on the cerebellum, we will recognise the paradoxical situation that in the cerebellum virtually every neuron is synaptically connected to every neuron, and yet the neuronal circuitry of the cerebellum functions in a meaningful way.
As a final preliminary consideration for the basic circuitry of the cerebellum, we need to hypothetically clarify how signal flow occurs from the mossy fibres to the Purkinje cells.
Apparently, the moss fibres are a sequential distribution system.
Therefore, we think of a cerebellum cluster as being composed of, say, n Purkinje groups. Each Purkinje group should receive the same input. Therefore, each Purkinje group should also be assigned exactly one granule cell for each mossy fibre, which taps into this mossy fibre within the spatial extension area of this group and forms an ascending axon, which branches in both directions in a T-shape in the molecular layer.
Now, if the length of these parallel fibres is so great that k Purkinje groups are reached from them, then these k Purkinje groups in turn also each have exactly one grain cell that taps into the same moss fibre. Therefore, each of these Purkinje groups will have a total of k parallel fibres, all derived from the same moss fibre. And the more Purkinje groups there are and the longer the parallel fibres become, the more parallel fibres receive their input from the same moss fibre. In this respect, the moss fibre system is a sequential signal distribution system for the Purkinje groups.
Theorem1.25 : The moss fibre system as a sequential distribution system
The moss fibre system distributes the cortex output sequentially to all Purkinje groups. Each Purkinje group receives input from each moss fibre via exactly one individual granule cell. However, due to the long length of the parallel fibres, each Purkinje group also receives input via the parallel fibres that originate from the same moss fibre in the neighbouring groups. Thus, the number of parallel fibres whose input comes from the same moss fibre is equal to the number of Purkinje groups that receive input from one and the same moss fibre. The propagation speed of the action potentials along the moss fibres is relatively high, so that all Purkinje groups receive the signal to be analysed (almost) simultaneously and can start the signal analysis (almost) simultaneously.
If a Purkinje group taps each moss fibre with exactly one granule cell, this granule cell may well form a larger dendrite tree, so that several (or many) synaptic contacts to the moss fibre are formed per granule cell (rosette-like tapping).
The parallel fibres to a certain Purkinje group can now be divided into 2 groups. We do this in a definition.
Definition1.24 : Primary and secondary parallel fibres of a Purkinje group
A parallel fibre whose grain cell lies within the area claimed by the associated Purkinje group and contacts a moss fibre there is called a primary parallel fibre of this Purkinje group. A parallel fibre that contacts a Purkinje group but whose grain cell lies outside the area occupied by this group is called a secondary parallel fibre.
With this we can establish another theorem.
Theorem1.26 : Projection of the cortex into a Purkinje group
Each signalling neuron of a cortex cluster projects into each Purkinje group via the mossy fibre system with exactly one primary parallel fibre. The remaining parallel fibres of this signalling neuron reach each Purkinje group as secondary parallel fibres whose origin lies in the neighbouring groups of the same cerebellum cluster.
Thus the cerebellum is formed by sequentially stringing together a basic element. This basic structure is the Purkinje group, the associated Golgi cell, the primary parallel fibres, the granule cells, basket cells and stellate cells belonging to the group, as well as the two nuclear neurons, one of which is excitatory, the other inhibitory. Furthermore, a Purkinje group includes a climbing fibre axon, which contacts all neurons of the group with the exception of the granule cells. In the case of the start group, this climbing fibre axon is the primary one, while all other groups are each assigned a secondary climbing fibre axon.
There is the possible variant that a granule cell could have synaptic contact with several, different moss fibres. The following mathematical principle of the imprinting of Purkinje cells is not rendered invalid by such a generalisation, but can be adapted to it.
Unfortunately, there is no indication in the available literature as to why there are basket cells and stellate cells in the cerebellum. Both types of neurons are excited by the parallel fibre input and both inhibit the Purkinje cells. While the basket cells are located in the lower part of the molecular layer and synaptically tap the parallel fibres located there, the stellate cells are located in the outer region of the molecular layer and restrict their synaptic contacts to this area. Because of the direct inhibition of the cell bodies of the Purkinje cells, the basket cells are often thought to have a stronger inhibitory effect. Star cells are thought to have more of a modulatory effect.
Therefore, the author assumes a certain division of tasks between star cells and basket cells. This will be stated in the following theorem.
Theorem1.27 : Division of labour basket cells and star cells
The signalling neurons of a cortex cluster project into the cerebellum via a primary mossy fibre population. The parallel fibre population derived from this mossy fibre population via the granule cells is synaptically contacted exclusively by the basket cells.
Another moss fibre population originates from the attention-regulating system (ARAS *) of the brain. The author counts among these the formatio reticularis as well as the magnocellular mean signals of cortex layer V, which ultimately find their way back to the cortex via the nucleus centromedianus (see resolution pyramid) and from there reach the moss fibres of the cerebellum via the bridge nuclei. The parallel fibres derived from this are contacted exclusively by the stellate cells.
* ARAS: Ascending Reticular Activation System
The parallel fibres that are contacted exclusively by the basket cells we will call direct cortical parallel fibres. The parallel fibres that contact exclusively the stellate cells we will call indirect parallel fibres. Similarly, we distinguish direct and indirect moss fibres depending on whether they feed the direct or indirect parallel fibres. Therefore, the following definition applies.
Definition1.25 : Direct and indirect moss fibres/parallel fibres
The mossy fibres that carry the cortical input from the cortex cluster to those parallel fibres that excite the basket cells and Purkinje cells are called the direct mossy fibres. We refer to the associated signal as the direct cortex signal. The parallel fibres that receive their input from the direct moss fibres are called direct parallel fibres.
We call the remaining moss fibres indirect moss fibres. They contact only the stellate cells and the Purkinje cells. We will call the associated signal the indirect signal and the parallel fibres the indirect parallel fibres.
Thus, the signalling neurons of the cortex feed the direct moss fibres and the direct parallel fibres with the direct cortex signal. The direct cortex signal ends at the basket cells and the Purkinje cells of the cerebellum.
The remaining neurons that project to the cerebellum feed the indirect mossy fibres and the indirect parallel fibres with the indirect cortex signal, which terminates at the stellate cells and the Purkinje cells of the cerebellum.
The meaningfulness of this dichotomy in the origin of the moss and parallel fibres remains to be seen. In any case, the parallel fibres of the stellate cell population are located further out in the molecular layer and are therefore presumably evolutionarily older. They belong to the magnocellular system, to which the formatio reticularis also belongs. In the author's opinion, the ascending formatio reticularis is a single-signal nucleus. A special feature is that there are several such single-signal nuclei in the ascending reticular formation, which also use different transmitters. The typical characteristic of such single-signal nuclei are the magnocellular single-signal neurons with their huge dendrite trees. In the "Functional Neuroanatomy" by Zilles /Rehkämper, 3rd edition, page 318/319 we read about this:
(start of quote:)
"The formatio reticularis extends from the mesencephalon to the caudal end of the medulla ablongata. The medial two-thirds consist of large neurons, the lateral third of small neurons." ... "The large neurons of the magnocellular zone have widely radiating dendrites that branch in a plane perpendicular to the long axis of the rhombencephalon. Thereby, the dendritic territories of the different neurons overlap strongly. Therefore, a single, large neuron can receive information from a large catchment area in the transverse plane and simultaneously from many fibre systems running perpendicular to this plane through the brainstem. This is the structural basis of the integrative function of the formatio reticularis."
(end of quote)
In view of the great importance of the formatio reticularis in the ascending activation system and for the indirect signal in the cerebellum, we summarise our findings in our own theorem, which we provide with the names of the inspirers.
Theorem by 1.28Zilles/Rehkämper: Single-signal neurons in the formatio reticularis
The excitatory large neurons of the magnocellular zone of the formatio reticularis form positive input signals as integration neurons, whose signal strength S increases with the strength of the integrated input. Here, different transmitters are assigned to different signal modalities.
Sketch: 1.25: The formatio reticularis - a positive one-signal nucleus
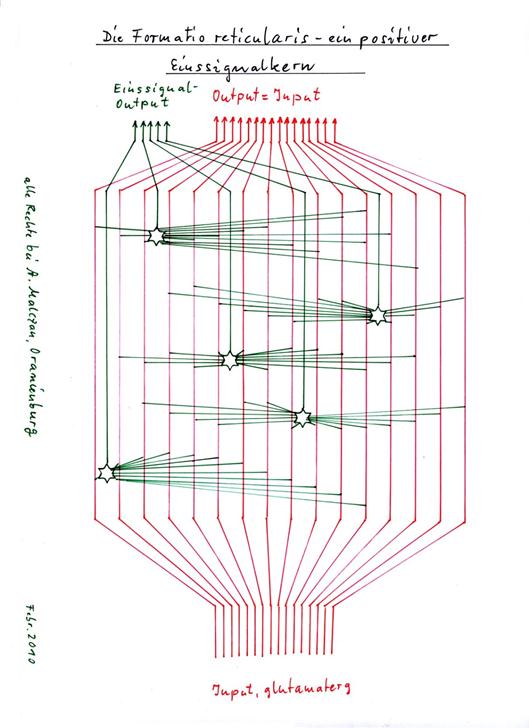
The existence of single-signal neurons in the formatio reticularis is mentioned for the first time in the 25-page monograph "Die neuronale Schaltung des Gehirns - Teil 1: Negationskerne" (The neuronal circuitry of the brain - Part 1: Negation nuclei) of 7 March 2010, which was also penned by the author of this monograph and sent to the publisher "Gehirn und Geist" (Brain and Mind), more precisely to Mr Hartwig Hanser at his request by e-mail on 7 March 2010 at 3.28 pm. On 20 April 2010, the author received notification from Mr Hanser that the publisher would not consider publishing it. From this, the author concluded that the quality of the work had not yet reached the necessary level. In fact, almost a year later, it turned out that some neuronal nuclei described in this paper as one-signal nuclei really did not have this property. The euphoria of having discovered real one-signal nuclei had led to counting too many neuronal nuclei as belonging to this class of nuclei. The criteria were apparently fulfilled, but these assumptions later proved to be in error. Other neuronal nuclei were only recognised much later as one-signal nuclei, negation nuclei or inversion nuclei.
While synaptic contact between climbing fibres and basket cells is generally recognised, evidence of synaptic contact between climbing fibres and stellate cells is less conceivable. The stellate cells are loosely distributed between the Purkinje cells. Nevertheless, we follow the explanations of Graumann/Sasse in "Anatomy" on page 280:
(beginning of quote)
"Climbing fibres contact stellate cells with their axon collaterals, these inhibit Purkinje cells in the sense of forward inhibition."
(end of quote)
After we have recognised that the climbing fibres contact basket cells, stellate cells, Golgi cells and Purkinje cells as well as the positive and negative nuclear neurons, we derive the only possible conclusion from this: All these cell types are contacted by the climbing fibres only so that the long-term depression or the long-term potentiation can be caused by the climbing fibre signals. A theoretical system analysis brings an even more precise result, which we summarise in a new theorem.
Theorem1.30 : The effect of the climbing fibre signal on cerebellar neurons
The climbing fibre signal causes a long-term depression in the Purkinje cells.
In the basket cells, the stellate cells, the Golgi cells and the positive and negative nuclear neurons, the climbing fibre signal causes long-term potentiation.
It would be a waste of material and labour for the climbing fibres to contact cells only to over-excite them with no further effect. Nevertheless, it should not be forgotten that nature need not be prone to senselessness. This is proven by the enormous variety of bony outgrowths, especially in the time of the dinosaurs. These were by no means always sensible. But under a certain evolutionary pressure, "sparingly" acting systems prove to be advantageous. Therefore, the climbing fibre contacts to all cerebellum neurons would probably have already atrophied if they had no "higher" significance. After all, enough time has passed, considerably more than was available to the dinosaurs at the time. Apparently, the climbing fibre contacts to all cerebellum neurons are necessary from a systems theory point of view.
With regard to the indirect mossy fibre signals, it must be mentioned that there are three subtypes of the cerebellum: the vestibulocerebellum, the spinocerebellum and the pontocerebellum. Here, these three subsystems differed in the origin of the indirect mossy fibre signals. The vestibulocerebellum receives its indirect mossy fibre signals (according to the author's unconfirmed opinion) from the formatio reticularis. This is also true for the spino-cerebellum. But the pontocerebellum receives (possibly in addition) the indirect mossy fibre signals from the layer V median neurons, which apparently not only draw to the nucleus subthalamicus, but (probably) also to the bridge nuclei. The indirect mossy fibre population terminates (in the author's opinion) predominantly in the stellate cell system of the cerebellum, where it has a very important role in controlling the working point of the associative matrices of the cerebellum. But more on this later.
We summarise the performances so far in an overview.
Theorem1.31 : Basic circuit of the cerebellum
The cerebellum consists of cerebellum clusters. Each cerebellum cluster corresponds to a cortex cluster. The signal neurons of the cortex cluster project via the bridge nuclei to the direct mossy fibres of the corresponding cerebellum cluster as well as to the positive and negative nucleus neurons of the same cluster. This signal represents the direct cortex signal; the projecting moss fibres contact only granule cells, which form synapses only with the basket cells and with the Purkinje cells and Golgi cells of the cerebellum.
The indirect moss fibres originate from the attention-regulating system (ARAS) of the brain - presumably the formatio reticularis - or the magnocellular mean system. Its signals reach the indirect moss fibres, which supply the indirect granule cells with input. Their axons form an indirect parallel fibre system that excites the stellate cells, but also the Purkinje cells, but not the basket cells. Whether this indirect signal reaches the Golgi cells is unclear, but rather unlikely.
The signal from the activity neuron of the cortex cluster reaches the cerebellum cluster via the striosome system of the basal ganglia as a primary magnocellular climbing fibre signal. The associated primary climbing fibre axon contacts exclusively the neurons of the first Purkinje group, which functions as the start group. All Purkinje cells, but also each basket cell and asterisk cell as well as the associated Golgi cell in this start group are contacted by the primary climbing fibre axon, as are the positive and the negative nuclear neuron of this start group.
The cerebellum cluster consists of a well-ordered series of Purkinje groups with a start group and an end group. Each Purkinje group except the end group has a successor group, and each Purkinje group except the start group has a predecessor group. The order of the Purkinje groups results from their spatial arrangement along the mossy fibres, which form a sequential rapid distribution system for cortex output.
Each Purkinje group ends with a Golgi cell, which in the case of excitation causes a double inhibition of conduction. On the one hand, it interrupts the propagation of the moss fibre excitation along the moss fibres to the following Purkinje groups. On the other hand, it interrupts the transmission of the excitation of the granule cells it reaches to their parallel fibres.
All Purkinje cells, the associated Golgi cell and all basket cells and stellate cells of the remaining Purkinje groups of the cerebellum cluster are each excited by an associated secondary climbing fibre signal when this is active.
The secondary climbing fibre signals are derived from the primary climbing fibre neuron of the olive either by central or sequential distribution in the olive.
The positive and negative nuclear neurons receive their single-signal excitation from all moss fibres and from the associated climbing fibre in the case of sufficient activity of the associated cortex cluster.
Each Purkinje group has exactly one positive and one negative nuclear neuron in the cerebellar nucleus. Both nucleus neurons are single-signal neurons and are inhibited by all Purkinje cells of the associated Purkinje group when they are active. The inhibition is relative and leads to an inversion of the Purkinje signal.
The positive nuclear neuron of each Purkinje group projects excitatoryly predominantly into the thalamus. The negative nucleus neuron of each Purkinje group projects inhibitory on the one hand into the nucleus olivaris inferior and suppresses the associated climbing fibre signal there in the case of activity. On the other hand, in the case of the nucleus dentatus, it projects inhibitively to the nucleus ruber, where it performs other tasks that have not yet been described.
The positive, i.e. excitatory, nuclear neurons of the cerebellar nuclei are linked to each other by inhibitory (glycinergic) interneurons, whereby each excitatory nuclear neuron inhibits the excitatory nuclear neurons in its vicinity. The strength of inhibition increases with increasing firing rate and decreases with increasing distance. We call such inhibition receptive neighbour inhibition. It leads to contrast enhancement of the signals. Receptive neighbour inhibition also exists in the thalamus and cortex cortex.
An indirect projection of the cortex provides an indirect cortex signal via the indirect moss fibres to those granule cells whose parallel fibres exclusively contact the star cells.
All Purkinje cells, the associated Golgi cell and all basket cells of a Purkinje group are excited by the primary and secondary parallel fibres when these are signal-carrying. The granule cells of the primary parallel fibres are located inside the associated Purkinje group, whereas the secondary ones are located outside.
The synaptic coupling strength between any two neurons of the cerebellum is co-determined by the occurrence of long-term potentiation or long-term depression.
Each parallel fibre reaches k Purkinje groups, each Purkinje group tapping into each moss fibre with its own granule cell, its axon forming the primary parallel fibre of the Purkinje group. Due to the long axon length of the parallel fibres, each Purkinje group ultimately has k parallel fibres whose input comes from the same moss fibre.
After this summary of all the essential details, we can explain how the cerebellum works. This also requires the use of mathematical approaches. Our aim is to prove that a cerebellum cluster forms a sequential chain of memory units. Each memory unit is realised by a Purkinje group and can store exactly one complex signal of the corresponding cerebellum cluster. Each significant complex signal is stored exactly once. Multiple storage is effectively prevented. The storage is fully automatic and autonomous.
Already now, a test criterion is to be established against which the correctness of the cerebellum theory, which is yet to be developed, may be tested.
In the basal ganglia, the evolutionary explanation of the acetylcholinergic projection of the matrix neurons into the striosome neurons was a touchstone. It could be shown that this projection originates from the time when there was no cerebral cortex at all.
It is known from the literature that it is very well possible to get along completely without a cerebellum. Anyone who was born without a cerebellum will hardly be noticed in normal life, if one believes the statements of various neurologists. However, he will only be viable if the nucleus ruber and the cerebellar nuclei are present. This shows that the nervous system can compensate for the complete absence of the cerebellar cortex.
However, if you were born with a functioning cerebellum, disorders in the cerebellum cause severe and sometimes life-threatening symptoms. Therefore, one cannot do without an already used and functioning cerebellum. Here, its failure can no longer be compensated for.
It is precisely on this feature that we want to test the cerebellum theory developed here. It will be shown that the nucleus ruber and the cerebellum nuclei are a functional subsystem in their own right, which can be interpreted as the "precerebellum". Only later, through evolutionary development, did this system receive the cerebellar cortex and thus became the complete cerebellum.
We now imagine the initial state of the cerebellum.
Let this be the case when the cerebellum has not yet learned a single signal,
i.e. is completely ignorant. We can describe this initial state hypothetically.
For this, however, we need some elementary insights into the synaptic coupling
strength between neurons.
Theorem1.32 : Theorem of forced coupling of compatible neurons in the primal state
When proneurons develop into neurons in a subsystem of the brain, transmitter-compatible neurons necessarily enter into a synaptic connection that is modified in the course of further development.
If the output neuron is LTP-capable, a minimum value k is minchosen as the initial value for the synaptic coupling and is later increased by the acting signals.
If the output neuron is LTP-capable, a minimum value k is minchosen as the initial value for the synaptic coupling and is later increased by the acting signals.
If the output neuron is LTD-capable, a maximum value k is maxselected as the initial value for the synaptic coupling and is later reduced by the acting signals. Theoretically, the initial values k min= 0.1 and kmax = 1 are useful (but also, for example, kmin = 1/2).
Justification:
Most neuronal systems undergo a development during the embryonic period and also postnatally, at the beginning of which there are so-called proneurons, i.e. the neuronal stem cells. The different types of neurons develop from them.
It makes no sense to develop proneurons into neurons if they do not establish synaptic contacts among themselves. Establishing contact among neurons is one of the most important tasks of nerve cells. Transmitter-compatible neurons must necessarily seek mutual connection. The pathfinding of the developing axons and dendrites is controlled by chemical substances - the so-called markers. These cause axons and dendrites to grow along the gradient of the marker concentration. Since the markers are partly produced by the axon tip and the dendrite tip themselves, both find each other.
Only the range of the markers - whose effect decreases with increasing "dilution" in the medium - is partly responsible for a limited range of the axons and den-drites and thus for the emergence of receptive fields. If necessary, the interested reader should consult the abundant international literature for more detailed information.
If LTP as a process leads to an increase in synaptic strength, it makes sense to start with the smallest possible synaptic value so that it can be increased later.
Similarly, it would be problematic to start with a too small synaptic coupling value when LTD becomes effective, since this is precisely what LTD is supposed to reduce.
As an example, we set the start values and the end values for neurological systems as follows:
- The minimum value k of minthe coupling strength for LTP-capable output neurons is kmin = 0.5. It may increase to the value k max= 1 through long-term potentiation.
- The maximum value kmax of the coupling strength for LTD-capable output neurons is kmax = 1.0. It may decrease to the value k min= 0.5 due to long-term depression.
Long-term potentiation thus increases the coupling strength from the value ½ to 1, while long-term depression decreases it from the value 1 to the value ½. These values are realistic example values for this monograph.
Furthermore, we must hypothetically clarify how the firing rate of a neuron is determined, which has n synapses with the coupling strengths k1, k2, k3, ..., kn , where the input neurons nhave the firing rates f1, f2, f3, ..., f and the neuron N1 with the firing rate f1 supplies synapse 1 with input, the neuron N2 with the firing rate f2 supplies synapse 2 with input, ..., and the neuron Nn with the firing rate fn supplies synapse n with input.
Definition1.26 : Firing rate of a neuron with multiple input suppliers in analogue mode.
Let N be a neuron in analogue mode.
Neuron N has n synapses, which are supplied with input by neurons N1, N2, N3, ..., Nn. With each input neuron, the output neuron has exactly one synapse.
Let K = (k1, k2, k3, ..., kn) be the coupling vector of the neuron N, where ki is the coupling strength of the i-th input neuron. Excitatory input counts positively, inhibitory input negatively.
Let F = (f1, f2, f3, ..., fn) be the associated vector of firing rates of the input suppliers, here let f be ithe firing rate of the i-th input neuron.
Then the firing rate fofN the neuron N results from the scalar product of the coupling vector and the firing rate vector divided by the number of synapses, if this number is non-negative, otherwise this firing rate is equal to zero
fN = K F */ n, if (K F */ n) ≥ 0 (normalised scalar product)
fN = 0, if (K F* / n) < 0.
With δ(x) = x for x ≥ 0 and δ(x) = 0 for x < 0 (Haeviside function) holds:
fN = δ[(k*f11 + k 2* f 2+ k 3* f3 + ... + kn * fn) / n].
Remark:
There are only non-negative firing rates as a result of the interaction of excitation and inhibition.
In addition to analogue mode, neurons also have binary mode and phase mode. According to the author of this monograph, the Purkinje cells in the cerebellum work in analogue mode.
definition 1.26only takes into account the well-known phenomenon that the more synapses a neuron has, the smaller the effect of a synapse. In these cases (at least simplified) the synaptic effect of a single synapse is indirectly proportional to the total number of synapses.
It is unclear whether this simple calculation method is close to reality. It assumes linearity for neurons in analogue mode. This is the strongest simplification in neuron models. However, it has the advantage of being easy to understand.
Now we can grasp the coupling strengths of the primordial state in the cerebellum more precisely.
Theorem1.33 : Coupling strengths of the primal state in the cerebellum
The Purkinje cells of the cerebellum are LTD capable.
The stellate cells, basket cells, Golgi cells and the nuclear neurons are LTP-capable.
The following table gives (as a rough approximation) the synaptic coupling strength k of each of these cell types to one of the neurons involved in the unprinted and in the imprinted state:
|
Cell type |
Unembossed |
Embossed |
Effect |
Type of embossing |
|
Moss fibre/pos. Core neuron |
kMF/KN+ = 0.5 |
kMF/KN+ = 1 |
arousing |
LTP |
|
Moss fibre/neg. core neuron |
kMF/KN-= 0.5 |
kMF/KN-= 1 |
arousing |
LTP |
|
Parallel fibre/Purkinje cell |
kPF/PZ = 1 |
kPF/PZ = 0,5 |
arousing |
LTD |
|
Parallel fibre/basket cell |
kPF/KZ = 0,5 |
kPF/KZ = 1 |
arousing |
LTP |
|
Parallel fibre/star cell |
kPF/SZ = 0,5 |
kPF/SZ = 1 |
arousing |
LTP |
|
Parallel fibre/ Golgi cell |
kPF/GZ = 0,5 |
kPF/GZ = 1 |
arousing |
LTP |
The remaining synapses in the cerebellum and cerebellar nuclei are not necessarily variable by LTP or LTD. They therefore have no climbing fibre contact and are constant in their coupling strengths according to the following table:
|
Cell type |
Coupling strength |
Effect |
|
Moss fibre/grain cell |
kMF/KZ = 1 |
arousing |
|
Golgi cell/moss fibre |
kGZ/MF = - 1 |
inhibiting |
|
Star cell/Purkinje cell |
kSZ/PZ = -1 |
inhibiting |
|
Basket cell/Purkinje cell |
kKZ/PZ = -1 |
inhibiting |
|
Purkinje cell/pos. Nuclear cell |
kPZ/KZ+ = - 1 |
inhibiting |
|
Purkinje cell/neg. nucleus cell |
kPZ/KZ- = - 1 |
inhibiting |
|
Moss fibre/pos. Core cell |
kMF/KZ+ = + 1 |
arousing |
|
Moss fibre/neg. core cell |
kMF/KZ- = + 1 |
arousing |
|
Climbing fibre/Purkinje cell |
kKF/PZ = 300 |
arousing |
|
Climbing fibre/stay cell |
kKF/SZ = 1 |
arousing |
|
Climbing fibre / basket cell |
kKF/KZ = 1 |
arousing |
|
Climbing fibre/Golgi cell |
kKF/GZ = 1 |
arousing |
(end of the theorem)
The synaptic effect of the climbing fibre on the Purkinje cell was (arbitrarily) rated at 300 because of the strong effect, since there are about 300 synapses of this axon with the Purkinje cell.
Likewise, the climbing fibre effect on the other interneurons of the (arbitrary) was set at 1.
At this point, the author is permitted to deviate more from the original first version of this monograph dated 15.08.2011. New insights required a rewrite of the entire Chapter 1.7 and a merger with the former Chapter 1.8. It is precisely these new insights that may now be explained.
In the course of the theoretical analysis of the coupling relationships in the cerebellum, the author noticed the division of labour between basket cells and stellate cells already postulated in Theorem1.27 .
In the above tables on the coupling factors, it is noticeable that there is an inhibition of the Purkinje cell by two cell types of the cerebellum. On the one hand, the Purkinje cells are inhibited by the basket cells. The latter have a larger dendrite tree. Similarly, the Purkinje cells are inhibited by the stellate cells. These have smaller dendrite trees. Both basket cells and stellate cells are excited by the parallel fibres.
This allows us to calculate the total synaptic coupling.
However, before we grasp imprinting - i.e. the learning process in the cerebellum - mathematically, we must clarify what an imprinting signal is supposed to be.
We start with the mathematical concept of the imprint vector.
Long-term depression and long-term potentiation in the cerebellum require the tetanic (higher frequency) excitation by the climbing fibre signal. But the parallel fibre signals must also have a certain and sufficiently strong excitation so that the synapse between them and the Purkinje cells, basket cells, Golgi cells and nucleus cells can be transferred from the unprinted primordial state to the imprinted state.
Therefore, we require that the signal-providing neurons of the cortex cluster in question have a relatively high firing rate in order to generate LTP or LTD, whereby the cell in question is imprinted and recognises this signal as its own. Let us call the necessary minimum value of the firing rate the imprint firing rate and prepresent it by the symbol f. Also necessary is a sufficiently long simultaneous exposure to a tetanic excitation via the climbing fibre as well as via the parallel fibres. We require such a minimum exposure time of one second.
The imprint firing rate thus determines whether a cortex signalling neuron with its output is included in the imprint signal of a cerebellum neuron or not. Therefore, we can specify the term imprinting signal. If a signal neuron does not achieve the required imprint firingp rate f, it cannot cause LTP or LTD in the associated parallel fibres.
Definition1.27 : (Binary) imprint vector of a signal vector
Let F = (f1, f2, f3, ..., fn) be the vector of the firing rates of the n (direct and indirect) signal neurons, where fi is the firing rate of the i-th signal neuron.
Furthermore, let f be pthe imprint firing rate.
Then we denote the vector consisting only of zeros and ones
P = (δ1, δ23, ... , δn)
as (binary) imprint vector, where the values δ1, δ23, δ, ... , δn are to be determined taking into account the imprint firing rate fp:
δ 1= 0, if f<1 fp, otherwise δ 1= 1, but only if f lasts at 1least 1 s
δ 2= 0, if f<2 fp, otherwise δ 2= 1, but only if f lasts at 2least 1 s
δ 3= 0, if f<3 fp, otherwise δ 3= 1, but only if f lasts at least3 1 s
.... etc...,
δn = 0 if f<n fp, otherwise δ n= 1, but only if f lasts at nleast 1 s.
The time measurement to determine the minimum duration of the signals involved starts simultaneously for all signal neurons. All signals whose duration is not at least one second from the start are dropped from the imprinting signal.
Thus, if the firing rate f of ithe ith cortex neuron is less than the imprint firing rate fp, the associated component in the (binary) imprint vector is equal to zero, otherwise equal to one, provided that this firing rate is realised for at least one second.
The minimum exposure time for the formation of LTP or LTD should be one second long here (as an example).
Example:
For simplicity, we choose only 5 neurons N1, N23, N4, N5. The corresponding firing rates are f 1= 200, f 32= 10, f = 70, f4 = 35, f 5= 110. Furthermore, let the imprint firing rate be equal to 50, i.e. f p= 50. Let each firing rate last at least one second.
Then the associated imprint vector P5 has the following appearance:
P 5= (1, 0, 1, 0, 1).
Because f1, f 3and f5 are greater than 50, and f as 2well as f4 are less than 50. Here, only 5 neurons were chosen so that the example remains clear. In the cortex cluster, however, there are several hundred ( or even thousand) cortex neurons.
For continuous signals, a definition of the imprint vector via a time integral would be possible. If the time integral of the signal strength of a signal neuron exceeds a minimum value over a time interval, the vector component of the imprinting vector would be equal to 1, otherwise zero. However, since the action potentials can ultimately also be simply counted as discrete events, the number of action potentials per time unit is equivalent. Equally almost equivalent to this is a minimum firing rate, as chosen here.
At this point, it should be briefly explained why the imprint vector has been given the attribute "binary". It has something to do with binary arithmetic, which we encounter predominantly in computers. It therefore makes sense to also assign a binary, or more precisely a dual number, to the binary imprint vector. Let it be formed by interpreting the individual components of the imprint vector as dual digits of a dual number. We will give this number a characteristic name and call it the signature of the imprinting signal.
Definition 1.28: Signature of an embossing signal
We call the dual number whose dual digits (respecting their order) correspond to the components of the imprint vector to an imprint signal the signature of the imprint signal.
The signature is a natural number. As a dual number, it has as many dual digits as the signal has components. Thus, the number of dual digits is equal to the number of neurons that make up this signal.
If the firing rate of the kth component of the signal is less than the embossing firing rate or does not last at least one second, the firing rate of the embossing signal is zero, likewise the kth dual digit is zero.
With the help of the (binary) imprint vector, we can now determine the imprint signal.
Definition 1.29: Embossing signal vector
The imprinting signal vector StoP a signal vector S results as the vector product of the (binary) imprinting vector P with the signal vector S:
P = (δ1, δ23, ... , δn)δ ifrom {0, 1} for all i
S = (f1, f2, f3, ..., fn).
S P= (δ 1*f1, δ f2*2, δ f3*3, ... , δ fn*n) (imprint signal vector).
Simplified, the imprint signal vector is derived from the signal vector by replacing all firingp rates smaller than the imprint firing rate with the number zero. Similarly, all firing rates that do not last for at least one second are set equal to zero. Here, the timing of the one second begins simultaneously for all neurons involved.
Our further procedure should follow a certain systematic. First, we calculate the signal ratios in the untrained cerebellum. This is when the cerebellum is in its original state and has not yet learned any signals.
We recall that the signals from the cortex cluster feed the direct moss fibres via the bridge nuclei, which in turn feed the direct moss fibres with input. From these, the direct parallel fibres are fed, which in turn excite both the Purkinje cells and the basket cells as well as the Golgi cells, but also the associated nuclear neurons. Thus, all these neurons are initially Dassigned the direct signal vector S, which consists of n signal neurons of the cortex cluster:
S D= (fD,1; fD,2; fD,3; ..., fD,n).
Here, fisD,i the firing rate of the i-th signal neuron of the cortex cluster. The signal strength of this signal is the mean value of the signal strengths involved:
f D= (f D,1+ f D,2+ fD,3 + ... + fD,n)/n.
The indirect signal vector Si consists of m components according to
S i= (fi,1; fi,2; fi,3; ...; fi,m).
The signal strength (rate of fire) of the indirect signal is given by
fi = (fi,1 + f i,2+ fi,3 + ... + fi,m)/m.
We form the vector of the total signal S as a vector sum, which now consists of n + m vector components, the first of which correspond to the direct signal vector and the last m to the indirect signal vector.
S = (fD,1; fD,2;D,3 f; ..., fD,n; f; fi,1; ...; fi,2i,3i,m).
The (theoretical) firing rate f is obtained by averaging over the (n+m) summands.
f = [(f D,1+ f D,2+ fD,3 + ... + fD,n) + (fi,1 + f i,2+ fi,3 + ... + fi,m)]/(n+m).
f = f/d(n+m) + f/i(n+m).
f =* pf D+ qf*i. (signal strength of the total input from direct and indirect signal)
Here p + q = 1 and p/q = n/m.
We do not count the climbing fibre signal here, as it is not to be learned. Only the current signal combination of direct and indirect signal is to be learned. The climbing fibre signal does act on the neuron to be imprinted, but only to trigger long-term depression or long-term potentiation. The more different signals a cerebellum cluster has learned, the more signals it will recognise and, in the case of recognition, suppress the climbing fibre signals. Thus, active climbing fibre signals become rarer and rarer in the course of time.
Now we recall the embossing process. Those components of a signal vector whose firing rate reached at least the embossing firing rate for the (as an example) given period of one second were assigned to the embossing signal. We assign the remaining vector components to the residual signal. Thus, the input vector can be represented as the sum of the imprint signal and the residual signal.
S = S P+ SR.
But since the signal S is already a vector sum of direct and indirect signal, the signal vector consists of 4 summands:
S = S DP+ S DR+ S + SiPiR.
The direct signal SD now consists of an imprinting part and an external signal part, the same applies to the indirect signal Si. It should be mentioned that all four vectors are linearly independent.
This input vector reaches the cerebellum via the mossy fibres. This is based on the simplifying assumption that each signal neuron of the direct cortex signal projects via exactly one bridge nucleus neuron to a moss fibre, whereby the firing rate may not change. The same applies to the indirect signal neurons of the formatio reticularis and the magnocellular mean system.
The easiest way to calculate the firing rates of the positive and negative nucleus neurons. Here we distinguish between the unprinted and the imprinted state of these neurons. The imprinting cause is the climbing fibre signal that contacts these neurons. For simplicity, we assume the following theorem:
Theorem1.34 : Unambiguous assignment of the climbing fibres to the nuclear neurons
Each climbing fibre contacts exactly one positive (glutamatergic) nuclear neuron of the associated cerebellar nucleus. Similarly, each climbing fibre contacts exactly one negative (GABAergic) nuclear neuron of the associated cerebellar nucleus.
Thus, the climbing fibres no longer contribute to the single-signal excitation of the nuclear neurons. They serve exclusively to imprint the nuclear neurons with the imprinting signal. We will see later that the climbing fibre signals are largely inhibited when signal recognition takes place. Therefore, we neglect their signal components in the calculation where it seems appropriate.
The firing rate of the unprinted nuclear neurons is equal to half the firing rate f of the total signal S. This is because each synapse of the cerebellar nuclear neurons has synaptic coupling strength ½ by default.
f=KN,u ½ f (firing rate of the unprinted nuclear neuron).
This is true because the individual signal components of the input vector
S = (fD,1; fD,2;D,3 f; ..., fD,n; f; fi,1; ...; fi,2i,3i,m).
with the coupling vector
K = (½; ½; ½; ...; ½) n + m components
multiplied scalarly. Each firing rate is multiplied by the corresponding coupling value ½, the sum is formed and divided by (n+m). The result is exactly half the firing rate f of the total input:
f=KN,u (½ f D,2D,1+ ½ f + ½ fD,3 + ... + ½ fD,ni,1 + ½ f + ½ f i,2+ ...; + fi,3i,m)/(n+m)
f=KN,u ½ f.
In the firing rate of the imprinted nuclear neuron, the synaptic coupling strengths are increased from the value ½ to the value 1 by LTP for the imprintable components of the signal. Therefore, the vector components of the imprinting signal vector enter the new firing rate with the value 1, while those of the residual signal vector are taken into account with the coupling strength ½. This total firing rate of the input vector had been decomposed into the direct and indirect components.
f =* pf D+ qf*i.
The input vector now consists of direct and indirect embossing signal and direct and indirect residual signal:
S = S DP+ S DR+ S + SiPiR.
The components of the direct imprinting signal S DPand also of the indirect imprinting signal SiP have at least the imprinting firing rate f over an imprinting period of one second (example value) and act on the nuclear neuron during imprinting simultaneously with the tetanic climbing fibre signal. As a result, the synaptic coupling strength increases from the previous value ½ to the value 1.
The synapses of the input axons to the direct and indirect residual signal retain their previous coupling strength of ½.
Multiplying out, summing and dividing by the number of synapses (n + m) gives the firing rate for the imprinted core neuron.
Each component of the imprint signal enters the resulting firing rate of the imprinted neuron with a factor of 1, the residual signal components with a factor of ½. Therefore, the following equation applies to the firing rate of the imprinted nuclear neuron
f=KN,g p * (f dP+ ½ fdR) + q * ( f iP+ ½ fi,R) (firing rate imprinted nuclear neuron).
f=KN,g ½ f + ½ fP
Here fP is the signal strength of the embossing signal, i.e. the average firing rate of the embossing component.
Theorem1.35 : Firing rate of the nuclear neurons
If f is the firing rate of the total input (without climbing fibre influence) and fP is the mean signal strength of the imprinting signal, an unimprinted nuclear neuron has the firing rate
f=KN,u ½ f (firing rate of the unprinted nuclear neuron),
while an imprinted nuclear neuron increases the rate of fire
f=KN,g ½ f + ½ fP (firing rate of the imprinted nuclear neuron),
owns.
From the above theorem, it can be deduced that the imprinted positive (glutamatergic) and negative (GABAergic) neurons of the cerebellar nuclei already send their imprinting signals with significantly stronger output towards the thalamus or olive or nucleus ruber.
Since there are only inhibitory interneurons in the thalamus, the neuron that receives the strongest input inhibits all neighbouring ones. Therefore, the strongest signal in the thalamus prevails and then informs the cortex that the corresponding nuclear neuron in the cerebellar nucleus has recognised its imprinting signal.
In contrast, the associated negative (inhibitory) nuclear neuron inhibits the climbing fibre signal in the olive or nucleus ruber and thus prevents the imprinting of other, free nuclear neurons with the already learned signal.
According to this insight, we want to call the area of the thalamus into which the positive nuclear neurons of a cerebellum cluster project the associated thalamic cluster.
Definition1.30 : Thalamic cluster
We refer to the area of the thalamus into which the positive nuclear neurons of a cerebellum cluster project as the associated thalamic cluster.
Therefore, the following theorem applies.
Theorem1.36 : The cerebellar nuclei as a storage system for imprinting signals
The cerebellar nuclei are also capable of learning without a cerebellar cortex and are able to learn imprinting signals through LTP. They are therefore comparable to memory circuits. The neuronal write command is the climbing fibre signal. The excitatory nuclear neurons inform the thalamus about the signal recognition, whereby, due to the receptive neighbour inhibition in the cerebellar nucleus and the associated thalamic cluster, the signals of the neurons whose imprinting signal has the best match with the currently applied signal prevail. The inhibitory projection of the negative nuclear neurons to the nucleus olivaris inferior inhibits the climbing fibre signals in the case of activity and thus prevents the multiple imprinting of signals in the cerebellar nuclei.
Now it becomes clearer why the cerebellum is not necessarily necessary for life. Those who lacked a cerebellum from birth but had functioning cerebellar nuclei and a functioning olive as well as a functioning nucleus ruber were able to lead an unremarkable life. According to leading neurologists, people with a completely absent cerebellum have often only been recognised during necessary medical examinations.
However, those born with a complete cerebellum can no longer easily compensate for the loss of the cerebellum. Serious complications and life-threatening symptoms are to be expected here if the cerebellum is massively damaged.
Nevertheless, the question arises as to why the cerebellar cortex came into being, when it functioned quite well without it. Here we need to go into further detail and analyse the contributions of the cerebellum neurons to the overall function.
Before doing so, for reasons of system theory and to avoid confusion, we should assign meaningful equivalents to the terms imprinting signal and residual signal.
Definition1.31 : intrinsic signal, extrinsic signal and signature of a neuron
If a neuron is imprinted with an imprinting signal by means of LTP, we call this imprinting signal the neuron's own signal. The part of any input signal for this neuron that does not belong to the intrinsic signal is called the external signal of this neuron. By imprinting, the signature of the imprinting signal becomes the signature of the intrinsic signal and the signature of this neuron.
Imprinting signal and residual signal are terms that specify the different input signal components of the cerebellum.
The intrinsic signal is a system property of a neuron that is induced by imprinting. Different neurons generally have different intrinsic signals and thus different signatures. This is related to the algorithm that prevents multiple imprinting of different neurons with the same imprinting signal in the cerebellum.
Now we can calculate the firing rate of the basket cells. These are capable of imprinting, are subject to long-term potentiation and receive only the input of the direct cortex signal. We neglect the possible signal strength of the climbing fibre signal.
Each signal neuron of the cortex cluster projects into a moss fibre. This supplies about w = 400 parallel fibres with its firing rate, which thus also becomes the firing rate of the w = 400 parallel fibres. These form w = 400 synapses with the basket cell per moss fibre.
But also the number of synapses of the basket cell is w = 400 times greater than the number of moss fibres. To calculate the firing rate of the basket cell, multiply the firing rates of the parallel fibres (which are equal to the firing rates of the associated moss fibres and thus equal to the firing rates of the cortex neurons) by the synaptic coupling strength and sum them up. The total sum is divided by the total number of synapses.
The unprinted basket cell has coupling strength ½ in each synapse. Therefore, for the firing rate fKO the equation applies
f KO,U= ( w* ½ f *D1+ w ½* f *D2+ w ½* f*D3 + ... + w ½ f**Dn) / ( w n*)
The factor w = 400 can be shortened. This gives the rate of fire of the unstamped basket cell as
f KO,U= ½ fD (the firing rate of the unstamped basket cell):
We see that the firing rate of the unprinted basket cell is identical to half the firing rate of the direct cortex signal.
This is logical to a certain extent, since each synapse of the basket cell has a coupling value ½, so that the cortex signals ultimately only affect the basket cell with half the total strength.
Now we calculate the firing rate of the basket cell after its imprinting with the imprinting signal.
Here, the direct cortex signal consists of the direct imprint portion S DPand the direct residual signal portion SDR.
S = S DP+ SDR
Before imprinting, all synapses of the basket cell had the coupling value ½. After imprinting by the climbing fibre signal, all synapses that were imprinted by the imprinting signal vector increased their synaptic coupling strength from the value ½ to the value 1. The associated change in the calculation formulae for the firing rate fofKO,G the imprinted basket cell are so simple that we only give the final result.
f=KO,G f DP+ ½ fDR.
f=KO,G ½ f D+ ½ fDP.
f=KO,G f+KO,U ½ fDP. (firing rate of the embossed basket cell)
Therefore, the following basket cell theorem applies.
Theorem1.37 : Basket cell theorem
Without the influence of the climbing fibre signal, the unstamped basket cell has half the firing rate of the direct cortex signal. The imprinting increases this firing rate by half the firing rate of the direct intrinsic signal of this basket cell.
The calculation of the firing rate of the unimprinted or imprinted star cell without the influence of the climbing fibre signal is analogous. However, the star cells only receive the indirect cortex signal of the attention-controlling system via their parallel fibres. Therefore, the indirect cortex signal takes the place of the direct cortex signal.
Because of the simplicity of this calculation, we only give the result.
f SZ,U= ½ fi (the firing rate of the unstamped star cell):
f=SZ,G f iP+ ½ fiR.
f=SZ,G ½ f i+ ½ fiP.
f=SZ,G f+SZ,U ½ fiP. (firing rate of the coined star cell)
Theorem1.38 : Star cell theorem
Without the influence of the climbing fibre signal, the unstamped star cell has half the firing rate of the indirect cortex signal. By imprinting, this firing rate increases by half the firing rate of the current indirect intrinsic signal of this star cell.
In the two theorems above, the terms direct intrinsic signal and indirect intrinsic signal were used. If the cortex signal consists of a direct and an indirect signal component, the intrinsic signal can also be divided into a direct and an indirect intrinsic signal component. The direct intrinsic signal component acts at the synapses between parallel fibres and basket cells, the indirect one at the parallel fibres and the stellate cells, whereby the intrinsic signal is necessary as input.
Next, we determine the firing rate of the unstamped and the stamped Golgi cells. We assume that these are only reached by the direct cortex signal, which is also true for the basket cells. And just like the basket cells, the Golgi cells are also subject to long-term potentiation, for which they have direct climbing fibre contact. Therefore, the firing rate of the unembossed Golgi cells is identical to the firing rate of the unembossed basket cells. Likewise, the firing rate of the embossed Golgi cells is identical to the firing rate of the embossed basket cells.
f=GO,G f DP+ ½ fDR.
f=GO,G ½ f D+ ½ fDP.
f=GO,G f+GO,G ½ fDP. (rate of fire of the imprinted Golgi cell)
Therefore, the following Golgizelle theorem applies.
Theorem1.39 : Golgizelle theorem
Without the influence of the climbing fibre signal, the unstamped Golgi cell has half the firing rate of the direct cortex signal. Imprinting increases this firing rate by half the firing rate of the direct intrinsic signal of this Golgi cell.
Now we calculate the firing rate of the unprinted and then the imprinted Purkinje cell. This receives the excitatory input of the direct and indirect cortex signal via the parallel fibres as well as the inhibitory input from the associated specific basket cell and the associated specific star cell.
Following an intuition, we decide to first calculate the excitatory input of the Purkinje cell. It results in a positive excitation firing rate. We then subtract the inhibitory input of the basket and star cells to determine the final firing rate. This is based on the assumption that the inhibition by the basket and star cells could completely inhibit the built-up positive excitation, so that the Purkinje cell becomes signalless.
We recall that according to this theory, the synapses of the parallel fibres with the Purkinje cell are subject to long-term depression. Therefore, the initial value of synaptic coupling before imprinting is equal to 1 and falls to ½ after imprinting.
Thus, for the firing rate of the unstamped Purkinje cell without the inhibition effect of the basket or star cell, the following equation applies
fPZ,U+ = ( 1 w* f *D1+ 1 w* f *D2+ 1 w *f*D3 + ... + 1* w f*Dn
+ 1 w *f*i1 + 1 w *f*i2 + 1 w* f*i3 + ... + 1* w f*im) / [ w (*n+m)]
The first sum represents the direct cortex signal transmitted by the parallel fibres,
the second the indirect cortex signal.
The factor w is truncated and we obtain
f PZ,U+= (f D1+ f D2+ fD3 + ... + fDn
+ fi1 + f i3i2+ ... + fim) / [ (n+m)]
However, this is exactly the expression for the total signal S and its firing rate f.
Thus, the firing rate of the unprinted Purkinje cell without the influence of the basket or star cell is identical to the firing rate of the total signal from the direct and indirect cortex signal.
fPZ,U+ = f = p f *D+ q f*i. (Fire rate of the unstamped Purkinje cell without the
inhibitory influence of basket and star cells).
From this we would now have to subtract the firing rate of the basket cell and the star cell, if we assume linearity for simplicity. In view of the fact that p + q = 1, we will let the basket cell act with the coupling factor p and the star cell with the coupling factor q, so that their total coupling also results in 1.
Then the result is
f=PZ,U p f *D+ q f *i- p ½* f D- q ½* fi.
It follows
f=PZ,U p ½* f D+ q ½* f i= ½ f.
f=PZ,U ½ f (firing rate of the unprinted Purkinje cell with consideration of
of the inhibition by the associated basket cell and star cell)
Thus, the unprinted Purkinje cell fires at half the firing rate of the total input when it is excited by the parallel fibres and inhibited by the unprinted basket and star cells.
Now we will calculate the firing rate of the Purkinje cell, which is exclusively caused by the excitatory influence of the parallel fibres after imprinting. We recall that the imprinting components of the imprinting signal at the synapses of the Purkinje cells lead to a significant reduction in synaptic coupling because long-term depression takes effect. However, it is precisely these synapses that are supplied with the imprinting signal. The coupling strength for the residual signal components remains at the value of 1, since neither the firing rate nor its duration of action are sufficient for imprinting.
The input for the imprinted Purkinje cell is provided by the total signal S from the direct and indirect cortex signal with the respective imprinting signal and the corresponding residual signal:
S = S DP+ S DR+ S + SiPiP.
Here, the following applies
S DP= ( fDP1; fDP2;DP3 ..., FDPK) direct embossing signal
n Components
S DR= ( fDR1; fDR2; fDR3; ...; fDRL) direct residual signal
S iP= ( fiP1; fiP2; fiP3; ... ; fiPm) Indirect embossing signalm components
S iR= ( fiR1; fiR2; fiR3; ... ; fiRn) indirect residual signal.
Each signal component now activates approximately w = 400 parallel fibres, which in turn excite the Purkinje cell.
In this case, the synaptic coupling for the imprinting signal has the coupling value k = ½, since the long-term depression has become effective due to the climbing fibre effect. For the residual signal components, the coupling value remains at the value 1, since the residual signal is too weak or too short to achieve long-term depression.
The following applies to the fire rate sought without basket and star cell impact:
fPZ,G+ = w (* ½ f *DP1+ ½ f*DP2 + ½ f *DP3+ ... + ½ f*DK +
+ fDR1 + f DR2+ fDR3 + ... + fDRL
+ ½ f*iP1 + ½ f*iP2 + ½ f *iP3+ ... ½ f*iPm
+f iR1 + fiR2 +iR3 ... + f iRn) / [ w ( *m + n)]
The factor w= 400, which corresponds to the number of parallel fibres per moss fibre, is again truncated here. It therefore remains
fPZ,G+ = ( ½ f *DP1+ ½ f*DP2 + ½ f *DP3+ ... + ½ f*DK +
+ fDR1 + f DR2+ fDR3 + ... + fDRL
+ ½ f*iP1 + ½ f*iP2 + ½ f *iP3+ ... ½ f*iPm
+f iR1 + fiR2iR3 + ... + f iRn) / [ ( m + n)].
fPZ,G+ = ( ½ f *DP1+ ½ f*DP2 + ½ f *DP3+ ... + ½ f*DK +
+ ½ f*DR1 + ½ f*DR2 + ½ f*DR3 + ... + ½ f*DRL
+ ½ f*iP1 + ½ f*iP2 + ½ f *iP3+ ... ½ f*iPm
+½ * fiR1 + ½ f*iR2 + ½ f*iR3 + ... + ½ f *iRn) / [ ( m + n)]
+ ( ½ f*DR1 + ½ f*DR2 + ½ f*DR3 + ... + ½ f*DRL
+½ * fiR1 + ½ f*iR2 + ½ f*iR3 + ... + ½ f *iRn) / [ ( m + n)]
The first four lines represent half the firing rate of the total input f, the remaining two lines represent half the firing rate of the residual signal vector:
fPZ,G+ = ½ f *+ ½ f*R. (rate of fire of the imprinted Purkinje cell without
Inhibition by basket and star cell)
Now we must subtractSZ,G from this rate of fire that of the imprinted basket cell KO,G fund of the imprinted star cell f.
We remember
f=KO,G ½ f D+ ½ fDP (firing rate of the embossed basket cell)
f=SZ,G ½ f i+ ½ fiP (firing rate of the coined star cell )
We subtract these firing rates from that of the imprinted Purkinje cell and obtain the resulting total excitation of the Purkinje cell:
fPZ,G+ = ½ f *+ ½ f *R- (½ f D+ ½ f DP) - (½ f i+ ½ f iP)
fPZ,G+ = ½ f *+ ½ f *R- ½ f D- ½ f - ½ f DP- ½ f iiP)
fPZ,G+ = ½ f *- (½ f D+ ½ f i) - ( ½ f DP+ ½ f iP) + ½ f*R
fPZ,G+ = ½ f *- ½ f - ½ f P+ ½ f*R
fPZ,G+ = + ½ f-R ½ fP (total firing rate of the imprinted Purkinje cell)
We recall that the firing rate itself cannot become negative. If in the above difference the subtrahend ½ f is Pgreater than the minuend ½ fR, then the firing rate of the Purkinje cell does not become negative, but the excitation supplied by the parallel fibres is smaller than the inhibition caused by the basket and star cells, so that the inhibition predominates. In this case, the Purkinje cell is finally inhibited and no longer fires at all. Therefore:
fPZ,G+ = + ½ f-R ½ f P if fR ≥ fP
fPZ,G+ = 0 if f R< fP.
Using the haviside function δ(x) with δ(x) = x for x ≥ 0 and δ(x) = 0 for x < 0, the following applies
fPZ,G+ = δ(+ ½ f-R ½ fP). (Total firing rate of the imprinted Purkinje cell)
We consider that fR was the mean signal intensity of the residual signal and fP the mean signal intensity of the imprinting signal. Through the imprinting, the imprinting signal of the cortex becomes the Purkinje cell's own signal. The corresponding residual signal component becomes the foreign signal component for this Purkinje cell. If, for any signal combination S, the Purkinje cell's own signal component is greater than or equal to the foreign signal component, the Purkinje cell completely ceases the previous inhibition of the associated positive or negative nuclear neuron. As the discoverer of this behaviour, which is mainly caused by the interaction of the long-term depression in the Purkinje cell and the simultaneous long-term potentiation in the associated basket cell or star cell, the author claims to give this phenomenon a self-chosen name. Since this behaviour is the basis for the work of the cerebellum as an associative memory and the theory of associative memories was already applied to the brain by Prof. Günther Palm in the previous century, this theorem shall be called Palm's theorem at the suggestion of the author.
Theorem 1.40: Palm's theorem: Inhibition path drop of a Purkinje cell
The inhibition of the output neuron of a cerebellar nucleus by the associated Pur-kinje cell is always omitted exactly when the intrinsic signal component of the non-trivial signal arriving from the cortex cluster is on average at least as strong as the remaining extrinsic signal component.
The requirement that the cortex signal be non-trivial is necessary because null signals also lead to the cessation of inhibition (e.g. death).
The above theorem surprised even the author of this monograph, who had hoped for years that each Purkinje cell, like the memory cell of a RAM memory, would only respond with a signal if its input was its own signal.
However, since an external signal component is now permissible, which may be at most as strong as the intrinsic signal component, the cerebellum is not a simple digital memory, but a digital search memory. The input to the search memory can be understood as a question. In general, the search memory gives several answers to a question, which, represented as a binary signal, correspond at least half to the input. But more about that later.
We will now calculate the output of the positive and negative nuclear neurons in the cerebellar nuclei. Here, the inhibitory firing rate of the corresponding Purkinje cells must be subtracted from the normal excitation of the nuclear neurons. This was shown in Theorem 1.35:
f=KN,u ½ f (firing rate of the unprinted nuclear neuron),
f=KN,g ½ f + ½ fP (firing rate of the imprinted nuclear neuron).
Furthermore, it was shown:
f=PZ,U ½ f (firing rate of the unprinted Purkinje cell)
fPZ,G+ = δ(+ ½ f-R ½ fP). (Total firing rate of the imprinted Purkinje cell)
From this we calculate the firing rate of the unprinted and the imprinted nuclear neuron belonging to this Purkinje cell and obtain:
f=KN,PZ,u 0 (total firing rate of the unprinted nuclear neuron)
f=KN,PZ,G ½ f + ½ f P- δ(+ ½ f-R ½ fP). (Total firing rate of the imprinted nuclear neuron)
Both results are remarkable. The unprinted nuclear neuron, regardless of whether it is glutamatergic or GABAergic, no longer has any firing rate at all. It is silent. It has a zero firing rate and its output is the zero signal.
In practice, however, we will find a weak spontaneous activity in these nuclear neurons, but this will have to be interpreted as "noise" on average.
However, the result for the imprinted nuclear neurons is also remarkable. Here it must be checked whether the intrinsic signal component of the Purkinje cell is at least as large as the residual signal component.
For the imprinted nuclear neurons, we recall the equation
f = f P+ fR, so also ½ f R= ½ f - ½ fP.
Used in
f=KN,G ½ f P+ ½ f R+ ½ f P- δ(+ ½ f-R ½ fP) = f P+ ½ f R- δ(+ ½ f-R ½ fP)
follows
f=KN,G f P+ ½ f R for fP ≥ fR
f=KN,G f P+ ½ fforP f P< fR
If fP ≥ fR, the minimum of f Pand f is equal Rto fR.
However, if f P< f Rholds, then the minimum of both numbers is equal to fP.
Therefore, we can also write:
f=KN,G f P+ ½ min*( fR; f P) (output of the imprinted nuclear neuron with consideration of the inhibition by the Purkinje cell and without climbing fibre influence)
Now we can summarise the firing rates of the different neurons of the cerebellar system for both the unimprinted and imprinted states. We call this summary the imprinting theorem of the cerebellum. We would like to point out here once again that we had simplistically assumed linearity, i.e. a simplified variant has arisen here.
The different significance of the direct and the indirect cortex signal will not be discussed. If time permits, it will be shown later that the indirect signal serves to regulate the sensitivity threshold of the associative memories. In relative rest (at high concentration), the indirect signal from the ascending reticular activating system becomes so weak that the associative matrix switches from question mode to memory mode.
Because the constant fraction ½ f in the excitation of the nuclear neurons is cancelled out due to the receptive neighbour inhibition by the glycinergic interneurons in the cerebellar nuclei, only the effective excitation fKN,eff of the nuclear neurons remains in the end.
Theorem 1.41: Imprinting theorem of the cerebellum
The firing rates of the unprinted and the imprinted cerebellum neurons satisfy the following equations under the condition of linearity, where f is the firing rate of the input signal and is composed of direct and indirect signal according to
f = f D+ fi.
Furthermore, let f be Pthe portion of the signal vector that has become imprinted, while fR represents the residual signal strength according to
f = f P+ f R= f+D,P f+D,R fi,P i,R.
|
Cell type |
Fire rate unstamped cell |
Rate of fire embossed cell |
Comment |
|
Nuclear neuron (pos. and neg.) |
fKN,U- = ½ f |
fKN,G- = ½ f + ½ fP |
without Purkinje impact |
|
Basket cell |
f=KO,U ½ fD |
f=KO,G ½ f D+ ½ fD,P |
direct signal |
|
Star cell |
f=SZ,U ½ fi |
f=SZ,G ½ f i+ ½ fi,P |
indirect signal |
|
Golgi cell |
f=GO,U ½ fD |
f=GO,G ½ f D+ ½ fD,P |
direct signal |
|
Purkinje cell |
f=PZ,U ½ f |
f=PZ,G 0 |
for f R< fP |
|
Purkinje cell |
f=PZ,G ½ f R- ½ f P |
for fR ≥ fP |
|
|
Nuclear neuron/ Purkinje cell (pos. and neg.) |
f=KN,PZ,U 0 |
f=KN,PZ,G ½ f + ½ fP - δ(½ f R- ½ fP) |
with Purkinje- influence, without receptive neighbour inhibition |
|
Nuclear neuron/ Purkinje cell/ glycinerge Interneurons |
fKN,eff = δ(f P- ½ max(fR, fP)) |
The constant portion ½ f cancels each other out |
End Theorem 1.41
In all data, the possible influence of the climbing fibre signal was neglected. The reason for this is that the climbing fibre signal is only active during the imprinting process, but after imprinting it is always suppressed by the negative nuclear neuron when the imprinting signal occurs again in the same cell.
The cerebellar memory is very different from the digital memory in ordinary computers. Its mode of operation also hides a different philosophy. The digital memory in a computer is organised sequentially and can only be written to and retrieved sequentially. For example, if you search for the word "car" in such a memory, you have to read out each individual memory cell and compare it with the search word "car". The result of the search process is the address of the memory cell in which the word "car" is stored. The same applies if you want to find out in the table of a database which customer with the name "Maier" works as a reporter and lives in Elsterwerda. Here, the table elements are queried one after the other and compared with the search values until there is a match.
The cerebellum is, as already indicated, a search memory. It can also determine the memory cell - more precisely, the Purkinje group - in which a certain intrinsic signal was stored. But the search process here is not sequential. The individual Purkinje groups are not queried one after the other, but all at once in one go.
While the searched signal - e.g. the word "car" - reaches the moss fibres via the bridge nuclei, it is distributed quasi simultaneously to all granule cells of the associated cerebellum cluster. Because of the enormous propagation speed of the action potentials along the mossy fibres, the search signal reaches all the granule cells almost simultaneously. Therefore, all Purkinje groups start evaluating the signal at the same time. Within a few hundredths of a second, each Purkinje group decides to what extent the search signal - i.e. the input - matches its own signal. The stronger the match, the more active the eigensignal detectors of the Purkinje group in question. These are the basket and star cells, but also the nuclear neurons and the Golgi cells.
At the same time, a check is made to see what percentage of the input signal belongs to the foreign signal of the Purkinje group in question. The more foreign signal content there is in the input, the more strongly the foreign signal detectors (i.e. the Purkinje cells) are excited.
The intrinsic signal detectors inhibit the extrinsic signal detectors so that there is a comparison of the signal strength of intrinsic signal components and extrinsic signal components. The result is the excitation of the nuclear neurons, which is stronger the stronger the intrinsic signal component was and which decreases with increasing extraneous signal strength.
All Purkinje groups whose intrinsic signal reached or exceeded the extraneous signal strength provide an output via the nuclear neurons, whereby a basic excitation of ½ f, i.e. half the input strength, is present even without a signal.
This basic excitation is eliminated by the inhibitory interneurons with the transmitter glycine, which are numerous in the cerebellar nuclei. Nevertheless, several, sometimes even many, nuclear neurons often remain active. This is due to the principle of the search grid. The question "Which words begin with the letter a?" yields many thousands of answers in the cerebellum within a few milliseconds, of which, however, only the strongest prevail. The principle of how these answers are cached in order to be named one after the other later will not be presented here. Please refer to Parts 3 and 4 of this monograph. What is important is the basic realisation that in the cerebellum, responses are not sought sequentially, but are delivered in a process that can be described as multitasking: All Purkinje groups of a cerebellum cluster work simultaneously and in parallel. And it is precisely this parallel mode of operation that is referred to as multitasking in conventional computers.
In addition to the multitasking capability of the many Purkinje groups, the ability to provide multiple or many answers to a search query forms another difference to the digital search memory.
The work of the cerebellum as a search memory can only be explained scientifically from a statistical point of view. If S is a search signal, which we feed into the cerebellum as input via the moss fibres, a digital signature belongs to this signal. For this purpose, the elementary signals are well-ordered and their firing rates are mapped to binary values, whereby stronger firing rates receive the binary value 1, the weak ones the binary value zero. This dual number represents the search input.
The task for the cerebellum is now: Determine all signals already stored that are sufficiently similar to the input. The more active moss fibres of the input signal excite the intrinsic signal detectors, the stronger the output of the corresponding nuclear neurons. And the more active moss fibres feed the foreign signal detectors, the weaker the output of the corresponding nuclear neurons. The cerebellum as a search memory thus makes a statistical statement about the signal similarity of input and stored cerebellum signals. The basis of the signal similarity are the bit positions assigned with 1 in the digital signature of input and output. The more of them match at the same position, the more similar two signals are.
In addition, the cerebellum has the amazing ability to automatically store a signal for which there are no sufficiently similar signals - i.e. which has not yet been stored - without a special external command (if this signal is sufficiently strong and lasts sufficiently long). The storage command that is nevertheless present - the parvocellular climbing fibre signal - it generates itself from the unsuppressed signal mean value of the associated cortex cluster (with the help of the striosome system). Therefore, the suppression of the climbing fibre signal in the case of signal detection has an extremely important functional counterpart: the non-suppression of the climbing fibre signal in the case of non-detection of a sufficiently active signal. And this algorithm allows an (unproven) extension: if strong signals are not detected, the climbing fibre signal is not suppressed. However, if there are no free Purkinje groups (because they have all been used up), the active and strong climbing fibre signal can imprint - i.e. overwrite - one of the already imprinted Purkinje groups with the new signal if this Purkinje group does not "resist" it. "Resisting" in this case means that this Purkinje group has "forgotten" its signal a little or even a little more. If a Purkinje group forgets its signal when it has not occurred for a long time, the weakening of the inhibition of the climbing fibre signal would then pave the way for a "new imprinting" or "re-imprinting" with a completely different, but statistically more frequent signal. This algorithm will be taken up again later in this monograph.
At this point, reference should be made to the concept of receptive neighbour inhibition, which has already been used several times. For example, when the cerebellum processes the input that is fed to it from the receptors on the surface of the body, the amount of input is enormous. Analogously, there is a huge amount of output. It is therefore extremely sensible in terms of systems theory to suppress the weaker output, whereby the stronger output gains contrast. For inhibition, it is sufficient if each output neuron inhibits the neighbouring output neurons via inhibitory interneurons. Stronger excited output neurons would thus result in stronger environmental inhibition. And since this inhibition decreases with increasing distance, we speak of receptive inhibition. It results in the emergence of the receptive fields in neuronal systems.
In the cerebellum, the receptive inhibition of the output is caused in two stages. Firstly, a neighbouring receptive inhibition is caused directly in the cerebellum nuclei via the glycinergic interneurons. And secondly, the excitatory cerebellum output reaches the thalamus. There are vast numbers of inhibitory interneurons there, which in turn favours the stronger signals but inhibits the weaker ones proportionally. Therefore, the receptive neighbour inhibition in the thalamus is an essential prerequisite to be able to evaluate the cerebellum outpout meaningfully at all. Receptive neighbour inhibition will prove extremely useful in many other areas.
Now we can understand the imprinting of the cerebellum as a recursive process in which one Purkinje group is imprinted step by step, one after the other. In this process, all Purkinje cells of a Purkinje group are imprinted with the same signal. But more on this later.
So we think of an imprinting signal SP, which acts on the cerebellum together with the climbing fibre signal. Initially, not a single Purkinje group is imprinted. The primary climbing fibre signal may provide the secondary climbing fibre signals by means of central or sequential distribution.
All nuclear neurons are unprinted and deliver the null signal. This null signal cannot inhibit the central distribution neuron; even with sequential distribution, inhibition does not occur anywhere.
Therefore, all secondary climbing fibre signals are identical to the primary climbing fibre signal and have a strong excitatory effect on all cells involved.
We start our result analysis with the Golgi cells. They are all contacted by the climbing fibres. Each Golgi cell at the end of each Purkinje group is strongly excited. It interrupts the transmission of the moss fibre signals on the moss fibres by its strong inhibitory effect. Only the start group, i.e. the first Purkinje group, receives the current moss fibre input, as the inhibition by the Golgi cell starts at the end of the start group, so that the moss fibres only become signalless after this start group.
At the same time, the Purkinje cells, the basket cells, the stellate cells and the Golgi cell of the start group receive the strongly excitatory tetanic climbing fibre input in addition to the moss fibre input. This imprints the Purkinje cells present in the start group of the Purkinje groups, as well as the basket cells, the stellate cells and the Golgi cells. But the Golgi cell is simultaneously excited by the strong climbing fibre signal and therefore inhibits the transmission of the moss fibre signals to the neighbouring Purkinje groups. Therefore, only the start group receives a moss fibre input. Since this, together with the tetanic climbing fibre input, acts for at least one second, all cells of this start group are imprinted. All other groups cannot be imprinted because the moss fibre input is missing. This is completely suppressed by the Golgi cell of the start group, which is always the last cell of this group at the end.
The signals of the secondary climbing fibres are identical to the signal of the primary climbing fibre, because they only emerge from it by distribution. But each secondary climbing fibre signal strongly and for a longer period of time excites the Golgi cell at the end of the associated group, so that any residual signals there are also completely suppressed. This would be possible if the Golgi cell of the start group had died in the meantime, for example. So no Purkinje group except the first can be imprinted with the incoming imprinting signal.
After the imprinting of the first Purkinje group, further signals arrive from the cortex. There are two possibilities here.
Either the signal is identical to the first imprinting signal. Then the Purkinje cells of the start group recognise their signal again. The associated positive nuclear neuron responds with a strong output to the thalamus. The associated negative nuclear neuron suppresses the central distribution neuron in the olive or the associated first neuron in the sequential distribution chain with its strong inhibitory output.
If the input signal contains at least half of the imprinting signal, the reaction is analogous. The thalamus is informed of the recognition and the negative nucleus neuron suppresses the generation of further climbing fibre signals to the other Purkinje cells.
After the imprinting of the starting group (or a subsequent group of Purkinje cells), however, the question arises as to why the Purkinje cell does not lose its imprinting signal again, which has become its own signal through the process of imprinting. This is because the Purkinje cells are still virtually overwhelmed with climbing fibre signals during central distribution and sequential distribution. Every stronger signal that has not yet been stored and is therefore not recognised leads to a strong climbing fibre signal. Because this signal is foreign to all previously imprinted Purkinje groups, each imprinted Purkinje cell will strongly inhibit its positive and its negative nuclear neuron in the cerebellar nucleus. Therefore, no negative nuclear neuron will be active to suppress the climbing fibre signal. Now, why is a Purkinje cell (or group) already imprinted with a different signal not "re-imprinted" from the old to the new cortex signal? After all, its climbing fibre signal is active if it does not recognise its own intrinsic signal, but no other Purkinje group recognises exactly this signal as its intrinsic signal either.
The solution to this open problem occupied the author of this monograph for several years. Here it was necessary to sift through the available facts, because only nature could have found a solution. The answer to the open question, why an already imprinted Purkinje cell is not imprinted during the activity of a hitherto unknown signal by the associated climbing fibre signal, is to be answered in a new theorem. The author takes the liberty of calling this theorem the Warsaw Theorem after the capital of his native Poland.
The first excited neuron inhibits the later excited ones.
Warsaw's theorem takes on special significance in the digitisation of analogue neuronal data. But its significance is already immense in the cerebellum. This will be explained in the following.
If a neuron - for example, the negative nucleus neuron in the cerebellum nucleus - is both excited and inhibited at the same time or with a small time interval, the resulting excitation is significantly less than expected. Often, nothing at all remains of the supplied excitation because inhibition completely predominates. Especially if the inhibitory axons are favourably placed - i.e. near the axon hill.
At this point, the work "The Brain" by Richard F. Thompson from Spektrum Akademischer Verlag should once again be cited with appreciation. On page 93 of the 3rd edition we read the easily understandable explanation:
(start of quote:)
Interaction between excitation and inhibition
The inhibitory postsynaptic potential has a stronger effect than the excitatory one. When an inhibitory synapse is active, it provides a short circuit, so to speak. The increased positive charge at the excitatory synapse can flow off there and the membrane at the axon hillock is consequently much less depolarised.
(end of quote)
The quoted text is preceded by an extremely easy-to-understand illustration that explains the facts.
Now, if the inhibitory effect is the first in time, while the excitatory effect sets in later, the end result is predictable: the neuron will remain inhibited.
How is this applicable to the cerebellum?
It is well known that the moss fibre signals from the cortex arrive in the cerebellum much earlier than the climbing fibre signal derived from the mean value. This is because the path of the mean signal of the activity neurons leads via the substantia nigra pars compacta, from there to the matrix of the striatum, on to the globus pallidus interna, on to the nucleus ruber and via the olive to the cerebellum. Therefore, a climbing fibre signal arrives in the cerebellum with a significant delay. In this time difference, the cortex signals have long since arrived in the cerebellum via the bridge nuclei. And any Purkinje cell that did not recognise its own signal had time enough to build up a strong inhibitory excitation.
The strong inhibitory excitation is passed on via collaterals within the same Purkinje group to the connected basket cells, stellate cells and to the Golgi cell.
Basket cell, star cell and Golgi cell are therefore very strongly inhibited and can no longer be imprinted by a possible incoming climbing fibre signal, because the long-term potentiation possible there requires an excited cell. With a strongly inhibited cell, no LTP will occur at all.
Since the Golgi cell is strongly inhibited at the end of the group, no forwarding inhibition is caused by it. The incoming moss fibre signals can therefore spread unhindered to the neighbouring groups.
It could be discussed whether the climbing fibre signal could have some effect on the strongly excited Purkinje cell, so that there would possibly be a long-term depression between just active parallel fibres and the Purkinje cell. In this case, the Purkinje cell would be a foreign signal detector for both the first imprinted signal and the second, third, fourth imprinting signal of the neighbouring groups. After very many imprints of the most diverse signals, the Purkinje cell would react to each of them with reduced excitation by half. However, this would not affect the function of the basket and star cells and the Golgi cells as intrinsic signal detectors. This is because these are certainly not being transduced, since the strongly inhibiting Purkinje cell effectively prevents further LTP. If now, with a certain time delay, the climbing fibre signal tries to excite the positive or negative nuclear neurons, these efforts come to nothing. The strong inhibitory influence of the Purkinje cell - which had not recognised its own signal - prevents an excitation effect through the synaptic "short circuit".
In this respect, the first excited neuron, in this case the Purkinje cell, inhibits the later excited neurons with which it is synaptically connected. Therefore, the strongly excited Purkinje cell, if it does not recognise its own signal, inhibits both the associated basket cell, the associated star cell, but also the Golgi cell at the end of the group as well as the nuclear neurons in the cerebellum nucleus.
The nuclear neurons therefore do not provide any output if an imprinted Purkinje cell does not recognise its own signal and a few moments later a strong climbing fibre signal tries to excite these neurons. Ultimately, the synaptic coupling of the climbing fibre signal to the nuclear neurons is not as strong as the synaptic coupling with the Purkinje cell.
So far we have explained the following:
- The Purkinje cells of the start group are the first to be imprinted with the first imprinting signal from the cortex cluster.
- During imprinting, the Golgi cell of this start group prevents the moss fibre signals from being transmitted to the neighbouring groups, thus preventing their simultaneous imprinting with the same signal. Excitation of the Golgi cells of the following neighbouring Purkinje groups by the climbing fibre signal has the same effect.
- When a new, different signal arrives from the cortex, followed by the associated climbing fibre signal, remoulding of the basket, asterisk and Golgi cells of the start group is effectively prevented because they are inhibited by the active Purkinje cell.
- It is theoretically possible to recast the Purkinje cell as a foreign signal detector.
- The output of the start group nuclear neurons during imprinting of other Purkinje groups with other imprinting signals is the null signal, because the inhibitory effect of the Purkinje cells of the start group preempts excitation by the climbing fibre signal and cancels this excitation by short-circuiting.
Now we have to show how the other Purkinje groups are imprinted with further, different imprinting signals. The imprinting of the first group has been shown. We show how the kth Purkinje group is imprinted when all its predecessor groups have already been imprinted with their own imprinting signal each. If this succeeds, we have shown in the sense of complete induction that all Purkinje groups in turn have each been imprinted with a different imprinting signal. This is exactly what we are now trying to do.
Let a signal flow from the cortex via the bridge nuclei and the moss fibres into the associated cerebellum cluster, which should not be the imprinting signal of the first (k-1) Purkinje groups.
- None of the first k-1 Purkinje groups recognise this signal as their own.
- Each Purkinje cell of groups 1 to k-1 is therefore strongly excited. It inhibits the Golgi cell at the respective end of the group, so that no forwarding inhibition of the moss fibre signals can occur.
- Therefore, the cells of the kth Purkinje group receive the complete cortex signal as input.
- At the same time, all Purkinje cells receive a climbing fibre signal when the primary climbing fibre signal is distributed to all remaining secondary climbing fibres using central or sequential distribution.
- None of the first k-1 Purkinje groups inhibit this climbing fibre signal at the distribution point because none of the associated Purkinje groups recognise their imprinting signal.
- The climbing fibre signal causes the strong excitation of the Golgi cell in the kth Purkinje group, so that this prevents the signal transmission on the moss fibres to the neighbouring groups. As a result, no Purkinje group after this kth group can receive moss fibre signals. Therefore, no imprinting with the current imprinting signal takes place behind the kth group, because although the tetanic excitation by the climbing fibre signal is present, most of the parallel fibres remain signalless. However, it must be considered here that the Golgi cells suffer long-term potentiation due to the imprinting by the climbing fibre signal. Therefore, their synaptic coupling to the intrinsic signal components is twice as strong as to the extrinsic signal components. Therefore, foreign signals are not as well suppressed and can very well work their way through, albeit weakened, to the neighbouring Purkinje group. Since there are already k-1 Purkinje groups in front of the kth Purkinje group under consideration, in which a new grain cell forms a new parallel fibre axon per Purkinje group, the kth group per moss fibre already has k parallel fibres of the same moss fibre. If this moss fibre is assigned to a foreign signal component, each Golgi cell inhibits this signal, but only half as strongly as the intrinsic signals. Due to the multiplication via the sequential distribution chain of the parallel fibres, foreign signals can therefore still become relatively strong in total. Therefore, a Purkinje cell that recognises an intrinsic signal cannot prevent the imprinting of the neighbouring, free Purkinje group with a completely different imprinting signal that simultaneously flows in from the cortex via the bridge nuclei. Only the imprinting of the same signal in the neighbouring groups is prevented!
- In the k-th Purkinje group, the strong tetanic excitation by the climbing fibre and the simultaneous excitation by the imprinting signal causes the imprinting of the cells involved: the Purkinje cells, the stellate cells, the basket cells, the Golgi cell and the positive and negative nuclear neuron are imprinted with the k-th imprinting signal. As a result, the Purkinje cells become foreign signal detectors, while the other cells become intrinsic signal detectors.
- In the kth Purkinje group, the Golgi cell at the end of this group is also very strongly excited by the incoming climbing fibre signal, causing a double inhibition of transmission. It interrupts the forwarding of the moss fibre input to the Purkinje groups following it as well as the forwarding of the moss fibre excitation to the granule cells it reaches. Due to the absence of the moss fibre signals, all Purkinje groups arranged after the kth Purkinje group cannot be influenced by the incoming climbing fibre signals, because the parallel fibre signals are missing.
- However, if the imprinting process of the kth Purkinje group is complete and the same signal continues to act unchanged via the moss fibres, the kth Purkinje group now recognises this signal as its own signal. The positive nuclear neuron of the kth group reports the signal recognition to the thalamus. The negative nuclear neuron immediately inhibits the climbing fibre signal and prevents any Purkinje group from being imprinted with this signal.
- If a signal arrives from the cortex that is not recognised as an intrinsic signal by any of the k already imprinted Purkinje groups, each of the first k Purkinje groups inhibits the Golgi cell at the end of the group. This removes the Golgi inhibition and the moss fibre signals reach the (k+1)th group, which is still unprinted. Since no climbing fibre signal is inhibited, the (k+1)th group is imprinted with this new signal if it is capable of imprinting, i.e. sufficiently strong and sufficiently long-lasting.
The basic functioning of the neurons in the cerebellum is based on the specialisation of the neurons in the course of imprinting by the tetanic climbing fibre input and the simultaneous action of the imprinting signals. Through long-term potentiation, the basket cells, the stellate cells, the Golgi cells, the positive and the negative nuclear neurons become intrinsic signal detectors. Their output to an intrinsic signal is significantly stronger than to an extrinsic signal. They also react as intrinsic signal detectors to a vectorial mixture of intrinsic and extrinsic signal components.
The Purkinje cells, on the other hand, react as foreign signal detectors after the imprinting process through long-term depression. Foreign signal components have a significantly stronger excitatory effect than intrinsic signal components. Through the additional inhibition of the Purkinje cells with the output of the self-signal detectors - in this case the basket and star cells - the Purkinje cell is completely inhibited exactly when the foreign-signal component of the Purkinje cell is less than or equal to the self-signal component of the basket and star cells. In this case, the foreign signal and the intrinsic signal balance each other out.
The whole thing is brought out of balance when the strength of the indirect signal approaches zero. Through "quiet contemplation", through concentration or the absence of disturbing stimuli, i.e. through a stress-free situation, the indirect cortex signal from the formatio reticularis (ARAS) is greatly reduced. However, the indirect signal was already present during the stressful imprinting process and therefore largely belongs to the intrinsic signal. If freedom from stress and inner peace now reduce the indirect intrinsic signal, the balance of intrinsic signal and external signal is thrown out of kilter to the detriment of the intrinsic signal. Therefore, the associative matrix suddenly delivers numerically fewer "answers". No longer do many similar signals provide recognition output, but only those that are strongly related to the direct cortex signal. The responses thus become more precise. This is precisely the process that is called "internal concentration" in learning. So the question: "Who prefers to eat meat and bones?" yields many answers under stress: dogs, wolves, jackals, lions, tigers.... etc. If the question is repeated later in a completely relaxed atmosphere, the answer is completely different: "Our dog Djego *) prefers to eat meat and bones!" Suddenly there is only one answer! Therefore, the ascending reticular system is not only of great importance in activating the cortex cortex (ARAS), but also in regulating the working point of the associative matrices of the cerebellum. The author believes that this finding is presented here for the first time in this monograph. But the inverse algorithm to this is also possible: the increase of sensitivity and response diversity through stronger indirect signals, e.g. through the mean signals of the pain system.
We put this insight into a theorem of its own.
*) Djego is the name of the dog of the author's family.
Theorem 1.43: The function of the indirect cortex signal in the cerebellum
The indirect cortex signal originates from the attention-controlling system and, in the standard case, becomes part of the imprinting signal and thus of the intrinsic signal of the Purkinje groups. If its strength later decreases due to external circumstances ("freedom from stress") or internal processes (concentration), the working point is shifted in the associative matrices of the cerebellum.
The shift is from question mode with relatively many answers to memory mode with relatively few answers. The resulting output signals are characterised by the fact that their foreign signal content is lower because the own signal content of the indirect signal has decreased as a result of the better concentration. As a result, the number of different output signals to an input signal decreases with decreasing signal strength of the indirect signal, i.e. the variety of responses becomes smaller.
Conversely, stronger indirect signals cause an increase in the response diversity and signal sensitivity of the cerebellum.
Similarly, a sudden increase in the foreign signal component, e.g. through unexpected noise (screams, thunder, gunshots), strong visual stimuli (lightning, rapid movement of large objects, fire), additional olfactory stress (smoke) produces a similar shift in the working point from the question mode with many responses to the memory mode with few responses, since then only those Purkinje cells respond whose own signal is so strong that it is not drowned out by the strong foreign signals. In extreme cases, there is no cerebellum output at all (not even a motor output!), the creature in question is then paralysed by fear and lapses into fear rigidity. So "playing dead" is not necessarily a decision born of intelligence, but a forced reaction to excessively strong foreign signal components in the moss fibre input of the cerebellum.
We summarise the special tasks of the intrinsic signal detectors and the extrinsic signal detectors in Cere-bellum in the following theorem.
The intrinsic signal detectors in the cerebellum have the task,
- recognise the intrinsic signal components (basket and stellate cells, Golgi cells, nuclear neurons)
- Inhibit the foreign signal detectors (Purkinje cells) in case of detection.
- report signal detection to the thalamus and thus also to the cortex (positive nuclear neurons)
- Inhibit the signal transmission of the intrinsic signal components along the moss fibres in the case of recognition (Golgi cells).
- To inhibit the signal transmission of the intrinsic signal components along the accessible granule cell axons to the parallel fibres within the same Purkinje group in case of recognition (Golgi cells).
- Inhibit the associated climbing fibre signal when the intrinsic signal components are detected (negative nuclear neurons inhibit climbing fibre neuron in olive).
The foreign signal detectors in the cerebellum have the task of
- recognise the foreign signal components (Purkinje cells)
- compare the mean strength of the extraneous signal components with the mean strength of the intrinsic signal components and determine the stronger signal by taking the difference between extraneous signal strength and intrinsic signal strength (Purkinje cells, inhibited by active basket and star cells)
- in the case that the foreign signal strength predominates, the transmission inhibition of the Golgi cells is prevented, so that the moss fibre signals can spread undisturbed to the neighbouring Purkinje groups (Purkinje cells inhibit Golgi cells)
- in the event that the foreign signal strength predominates, the basket cells, stellate cells and Golgi cells and nuclear neurons, which act as intrinsic signal detectors, are very strongly inhibited by the associated foreign signal detectors (the Purkinje cells) in order to prevent a possible remoulding by an incoming climbing fibre signal
- in the event that the extraneous signal strength predominates, the output of the negative nucleus neuron is strongly inhibited. As a result, this nuclear neuron can no longer inhibit the associated distribution neuron in the nucleus olivaris. Its output, in turn, can be used as a new climbing fibre signal for the neighbouring Purkinje group. If this group is still unprinted, it is now imprinted with the applied signal if the signal strength is sufficient.
Now it is finally clear that the neurons of the cerebellum represent a well-tuned signal-processing system whose task is the automatic storage of essential (i.e. imprintable) cortex signals.
The overall circuitry of the cerebellum using the example of two Purkinje groups, each consisting of three Purkinje cells, is shown in the sketch below.
Because of the shortness of the axons, the inhibitory output of the basket and star cells becomes effective promptly. It should be noted that the (longer) inhibitory axons of the Purkinje cells should be fast conducting, as the cerebellar circuitry does have time-critical behaviour.
Sketch1.25 : The circuit of the cerebellum
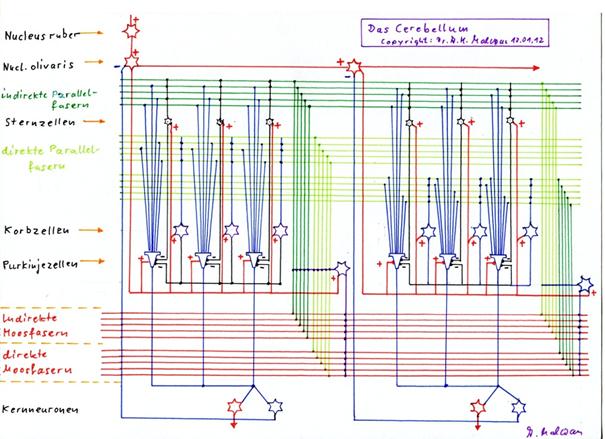
(The inhibitory axon collaterals of the Purkinje cells to the basket, star and Golgi cells have been omitted for clarity).
For non-mathematicians, it will of course be somewhat more difficult to understand why a Purkinje cell recognises an intrinsic signal, although it can be superimposed by a foreign signal component. The cerebellum works as a search memory, so to speak.
A search memory answers questions. The input of the search memory is a signal vector that is perceived as a question. The output neurons of the cerebellar nuclei provide the answer of the search memory. They are always excited when the intrinsic signal of the associated Purkinje cells corresponds at least halfway with the input signal.
Example:
On the input "house", for example, a search grid delivers the following terms as output:
- Front door
- House roof
- Bauhaus
- in the house
- ...,
It is characteristic that the input consists of 4 letters, whereas the output consists of a maximum of 8 letters. This means that the input "house" matches the output by at least 50 %, i.e. it contains at least half of the embossing signal.
Decades ago, the presumed correlations of the input vector and output vector of the Purkinje cells (but without taking into account the basket and star cell fraction!) were summarised in a matrix that went down in history as the "associative matrix".
At this point, I would like to thank Professor Günther Palm for his interest in the author's ideas, but much more for his successful efforts to point out to the author the connection between the cerebellum and the theory of associative memory. Only then did the author really understand why a Purkinje cell responds to an input of which it had stored only "half" of the signal, so to speak. Günther Palm has been successfully researching the development of neuronal intelligence for decades.
The theoretical model of the author of this monograph extends his theory of associative memories in that now the climbing fibre signal occurs as a write command and replaces Hebbian learning, whereby the learning process for a single signal can be reduced to the duration of one second (example value). Secondly, new signals are constantly added to the associative matrix, i.e. its number of columns is constantly increased, until the number of unprinted Purkinje cells of the cerebellum cluster is used up and no new ones can be formed. And finally, the author's model reveals the origin of cerebellum input. Particularly gratifying is the demonstration that the many millions of cortex clusters each form their own associative matrix in the cerebellum and all work in parallel.
We summarise the principle functioning of the cerebellum as a neuronal store for complex signals using the magnocellular climbing fibre signals in a separate theorem.
Theorem 1.45: The magnocellular climbing fibre signal as a memory command of the cerebellum
The primary magnocellular climbing fibre signal, which is derived from the activity neuron of a cortex cluster by the striosome system of the basal ganglia, and the other secondary magnocellular climbing fibre signals derived from it form a class of neuronal write commands of the cerebellum. They cause the forced storage of the imprintable signals applied to the parallel fibres during their activity in the next free Purkinje group. Thus, by means of these climbing fibre signals, the cerebellum stores the imprintable complex signals occurring in the associated cortex cluster, whereby a Purkinje group is assigned to each imprintable complex signal. If an already stored complex signal later occurs again as an input of the cerebellum, it is recognised by the Purkinje group whose imprinting signal it was, provided that the foreign signal component is not stronger than the intrinsic signal component.
At this point at the latest, the question arises as to what the higher subsystems of the brain do with the output of the many primary cortex clusters. It can be assumed that this output is further processed in the associative areas of the cortex cortex.
In this respect, the main task of the striosome system and the cortex clusters is obviously to first determine the essential signal combinations and define them as independent signals.
Although it may seem to us that we have understood the function of the cerebellum, we are still only at the beginning of our chain of knowledge. The question now arises as to which signals the cerebellum actually processes and which elementary signals are combined in it to form new complex signals.
So far, we have only analysed the effect of climbing fibres provided by the magnocellular system of the brain. Here, the striosome system of the basal ganglia was significant. It provides the neuronal system clock, the first indication of computer-like working. And it provides the magnocellular climbing fibre signal as a neuronal write command. This is the second clue to computer-like functioning. Now it is time to analyse the other climbing fibre systems of the mammalian brain. Here, further important insights are in store for us.
ISBN 978-3-00-037458-6 ISBN 978-3-00-042153-2
Monografie von Dr. rer. nat. Andreas Heinrich Malczan