Theory of Neuronal Circuitry in the Brain
and analytical cognition
ISBN
978-3-00-045141-6
Monograph by Dr. rer. nat. Andreas Heinrich Malczan
Chapter 3: The limbic system
Dedicated to James Papez, the neurologist whose opinion that the structures of the limbic system are linked to each other by a conduction chain of high-capacity circuits inspired this part of the author’s monograph. The Papez Circuit, which has reaped much praise but has also much criticism, is the theoretical basis used to explain signal processing and information management in the limbic system
The Oranienburg prophesy:
It is common sense, and not computer simulation, that will enable us to broaden our knowledge of the world!
(Andreas Heinrich Malczan - June 2013)
Section 3.1. An excursion into the primeval ocean of epochs long past
Let us risk an excursion into the murky depths of times long past, when the first animal species were working out ways of actively obtaining their food. Let us select one of these primitive animal organisms and take a closer look at it. To facilitate identification, we can call him Fred.
Like all living things Fred tended to get hungry. But food availability was not a problem; he lived in the primeval ocean where food was available in abundance. The ocean was enormous and deep and its depths were pitch dark. Fred had never seen what we know as daylight. But this was not just because it was dark. The shallower parts of the ocean near the coasts of the land masses were actually quite bright. The problem was that the primitive animals of that epoch had not yet developed eyes. At the very most, they may have had a few photosensitive skin cells enabling them to distinguish between light and dark. But they could not actually see anything. Creatures like our Fred had never seen daylight because they were blind. It was with his sense of smell that Fred located his food. He was not herbivorous, but he depended on herbivores for his survival; they were his prey. But there was one problem. When Fred got a whiff of something that smelled good, it nearly always swam away. Because the animals on whom Fred preyed were mostly good swimmers. And that was not all; almost as soon as Fred had smelled his prey, he forgot about it.
Fred’s olfactory receptors were excited when the prey swam past. The resulting action potentials were transmitted to a neuron layer that would later be described as the olfactory cortex. As Fred’s muscles were now activated via motoneurons by the transmitter substance acetylcholine, the axons of the cortex had to be switched over from glutamate to acetylcholine. This was done by a transactional core which would later be named the septum. The signals switched over to acetylcholine in the septum were transmitted to the motoneurons allotted to them in the muscle groups, and these generated Fred’s mobility and controlled his steering function.
It obviously made sense to activate Fred’s whole system, not just the muscle groups responsible for the actions. That is why modern-day vertebrates still have cholinergic projections from the core of the septum to the hypothalamus, the part of the diencephalon controlling not only the autonomic nervous system but also the nucleus suprachiasmaticus which, in turn, controls the body’s circadian rhythm. Similarly, the septum projects via the habenula to the monoaminergic cores in the tegmentum, i.e. to the dopaminergic VTA, the adrenergic locus coeruleus and to the serotonergic nuclei raphes magnus. The transmitters in the two last-named organs activate the prosencephalon in vertebrates and mammals. And it is still the habenula in present-day animals which integrates the olfactory, septal and amygdaloidal signals and transmits them via the nucleus dorsalis tegmenti to the neuron nuclei responsible for activating an animal’s masticatory and deglutitory muscles after it has killed its prey. All these systems are, moreover, present in duplicate. Work sharing between the left and right halves of the body was a relatively early evolutionary development.
Consequently, activation of the muscles and the neuronal systems by olfactory perception when either prey or enemies made their appearance was a step in the right direction. It was actually quite a good strategy, as long as the prey or the predators were slow to react and sluggish in their movements. But evolution did the rest. Many prey animals optimized their systems and escaped being eaten by reacting more quickly. The result was that they multiplied rapidly. And displaced the slower species which fell victim to the predators.
This gain in speed created a new problem. There was no parallel increase in the rate of diffusion of the scent emitted by the prey and this tended to decline sharply as it dispersed. This meant that, although perceptible, the scent of a rapidly moving prey would be located at a point where the prey no longer was and, in any case, it quickly evaporated. So the information received by the predators was extremely short-lived and the sensory receptors quickly closed down again. Fred had lost the scent of his prey.
It seems highly probable that Nature’s initial solution to this problem will have been enhancement of the sensitivity of the olfactory receptors and evolution of the sense of smell to a remarkably high level of efficiency. But this was nullified by further increases in the prey’s reaction speed, which frequently enabled it to escape the predator’s fangs. And its scent soon disappeared.
This meant that predators not wishing to die of starvation had to develop a memory – some sort of nerve cell circuit to remind them that a tasty morsel had just swum by at high speed. This memory would enable the predator to pursue that prey or search for other prey that had found itself a hiding place. The reminders emitted by this memory would generate action potentials that would ultimately control the muscle groups used for pursuit of prey.
The predator’s sense of smell provided the input for these signals. Specifically designed receptors – olfactory cells – generated action potentials when the right kind of prey swam by. These receptors could detect the metabolites produced by the prey and diffused through its skin – even under water. Of course, the same applied to the hungry predators, whose greedy, wide-open jaws were disgorging similar metabolites and betraying their presence. This is the reason why an animal’s sense of smell was and had always been so important and so incredibly sensitive. It was equally important for procreation of the species. Even in Fred’s day, sexual attractants, now called pheromones, were already the agents responsible for bringing male and female of the same species together for the purposes of sex and procreation.
There was no lack of prey in those days. The only problem was that Fred forgot about it as soon as it had swum past him; the scent molecules were quickly dispersed in the ocean’s enormous mass of water. The signals stopped as soon as excitation of the olfactory cells ceased. The prey was forgotten. What needed to happen?
Some brain mechanism that repeated the signals had to evolve. Something akin to the forgetful housewife, who keeps murmuring “We’re out of coffee, we’re out of coffee”, until she finally notes it down on her shopping list. The technical solution was repetition!
It was a new neuronal subsystem functioning as an echo generator that produced this constant repetition of the original signals. This generated a stream of echoes from each input that was persistent enough to achieve the same result as the forgetful housewife’s addition to her shopping list. The original input came from the olfactory cortex. The ensuing output was a series of echoes repeating the input signal as often as was necessary to achieve the desired result.
Each of the output neurons in the olfactory cortex had its own output axon, through which Fred’s physical functions were activated via the septum when his olfactory cortex was excited by the action potentials signaling the presence of prey. The autonomic nervous system was responsible for activation and deactivation of these physical functions. But how did Nature solve the forgetfulness problem? What sort of repetition could provide the technical solution? What sort of circuit would provide a suitable echo generator?
The action potentials of each output neuron in the olfactory cortex were also conducted to a new neuron with a considerably longer axon. This could best be described as a transactional neuron. During the course of evolution this mass of tiny transactional neurons formed its own layer, in which they were embedded as granules. That is why they are now called granule cells. The axons of granule cells were not myelinated and this meant that each action potential was transmitted relatively slowly, for example, at a rate of only 0.2 millimeters per millisecond. That is equivalent to 0.2 meters per second – roughly the same speed as present-day action potentials transmitted along unmyelinated fibers. This speed will certainly have been slower back in Fred’s day, simply because there was not yet any need for anything quicker.
If an axon of this type was twenty millimeters long, an action potential took 100 milliseconds to cross it. This caused a time lag. In physical terms, the axon was a conductor delaying the action potential travelling along it. For example, if this conductor was tapped 40 times at different points and at roughly equivalent intervals, forty echo signals would be received at intervals of 2.5 milliseconds. But there was as yet no neuron group to do the tapping, so one had to be evolved. These forty echoes were conducted to an integration neuron which put together a new series of signals containing the forty echoes. This integration neuron was also a new type of neuron playing a key role in the evolving echo generator.
From now on we will call these neurons doing the tapping ‘echo neurons’. In our example they tap the delaying conductor to produce forty echoes spread over a period of 100 milliseconds. We will call these primary echoes. After their collection in the integration neuron a secondary echo of considerably longer duration is produced.
This creates a memory lasting one hundred milliseconds overall – the time taken for the action potential to be delivered in the form of output from the final echo neuron. An integration neuron has collected the forty separate echoes and used these to produce a time-lagged output signal, which was a secondary echo of, for example, 100 milliseconds’ duration at a firing rate of approx. 400 Hz.
If, however, the transmission speed of the action potentials had been only 0.1 m/s instead of the 0.2 m/s assumed in our example, the secondary echo would have lasted 200 milliseconds. The drawback was that, in order to reach a firing rate of 400 Hz, it would have been necessary to tap the delaying conductor 80 times. But even an echo lasting 100 milliseconds was better than nothing. Fred would at least be able to remember for that length of time that a potential prey had just swum by, and receive forty reminders of this from the 40x duplication of the original action potential. This was a memory of sorts, even if it was extremely short.
Animals using this kind of echo generator were able to remember the prey longer, but it must be assumed that this type of system was not particularly efficient in its early days. At the start there would perhaps be only one single echo neuron, but even this would double the number of action potentials generated from one olfactory excitation, as compared to a system producing no echoes at all. And the number of echoes went one higher with the inclusion of each additional echo neuron to tap the conductor. As each action potential was an electrical impulse, the number of these impulses multiplied accordingly. Electrical engineers nowadays call this frequency multiplication. It is a principle that was perfected in neurological systems many millions of years ago.
All the technical elements of the neuronal echo generator now have their own names. The axons functioning as delaying conductors are called mossy fibers. The neurons belonging to them are granule cells. The axons responsible for rapid transmission of the echoes from the action potentials to the integration neuron are called Schaffer collaterals, named after the famous neurologist Károly Schaffer (1864-1939) who did most of his work in Budapest. The neurological structure of echo generators in a modern mammal’s brain is called the hippocampus. The Latin name is Cornu Ammonis (CA for short), in English Ammon’s horn. This is subdivided into four serially numbered sections. Section 3, the CA3 region of the hippocampus, contains the echo-generating neurons, which are called CA3 pyramidal cells because of their shape. The CA1 section contains the integration neurons which unite the many separate echoes received from the mossy fibers into a secondary echo. These neurons are called CA1 pyramidal cells.
The degree of freedom of interpretation possible in any description of the circuitry of neuronal echo generators is quite astonishing. Mathematicians and electrical engineers will tend to visualize a delaying conductor of this kind as a simple conductor forming a tiny branch at each echo neuron, with the echo neuron tapping the conductor at this branch. Each echo neuron has its own output conductor to the integration neuron responsible for collecting the individual time-lagged echoes.
Fig. 1: Simplified diagram of an echo generator
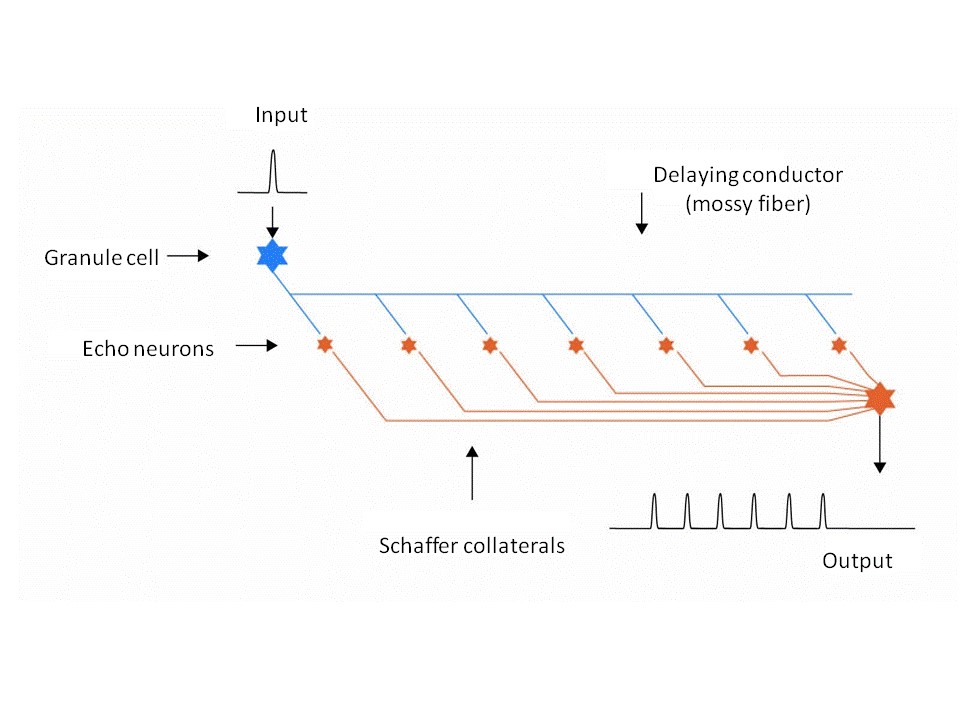
Neurologists will take a totally different view of the same set of facts. They know that the CA3 pyramidal cells in the hippocampus are quite large neurons which (according to the author’s theory) should function as echo neurons. A single synapse is not capable of generating an action potential in the mossy fiber acting as delaying conductor. Consequently, the relevant mossy fiber, like nearly all axons, creates a dense network of axodendrites that either surrounds it or completely replaces it. These axon structures in the hippocampus are called mossy fibers because of their moss-like network structure; they have to be elongated because it is their length that enables them to function as delaying conductors. And an echo neuron – those in the hippocampus are called CA3 pyramidal cells – now taps this ramified axon structure containing hundreds (or even thousands) of synapses, which together transmit an excitation that is sufficiently strong to generate an action potential.
But the axon conductors of the CA3 pyramidal cells functioning as echo neurons, which are called Schaffer collaterals, also form their own telodendrons on the C1 pyramidal cells functioning as integration neurons; these also have large ramified telodendrons making countless synaptic contacts with these equally large axodendrites. This type of Schaffer collateral is extremely numerous and they tend to run parallel to each other to form a network of nerves called a plexus. The result is that echo generators have possess some marked characteristics that make them readily identifiable.
Fig. 2: More realistic diagram of an echo generator
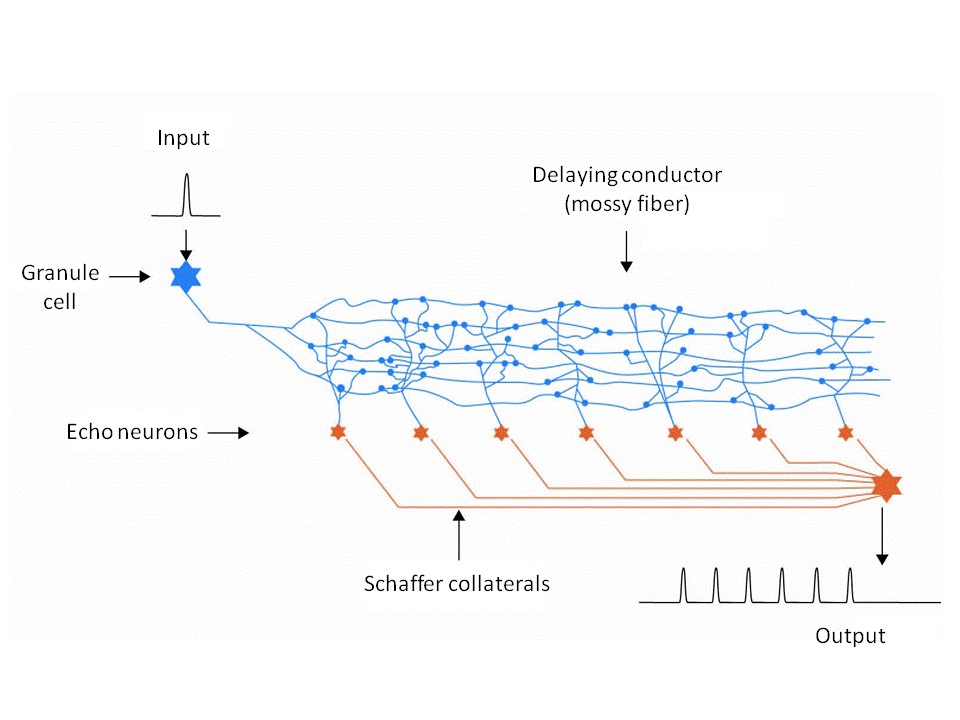
One advantage of a low diffusion speed of the action potentials along the delaying conductor was that this stretched the duration of the echo signals. This prolonged an animal’s memory of its prey and enabled it to continue the pursuit. Animals developing this faculty won the evolutionary race. But once this type of echo generator had evolved in all surviving animals of this subphylum (i.e. vertebrates), this evolutionary advantage was effectively neutralized. Predator and prey once again had equal fighting chances. But some still had a slight edge over the others by using low-speed action potentials along the delaying conductors to maximize echo generation. Further stretching of the prey’s echo would have been advantageous, because this would enable the predator to seek out a prey that had managed to hide itself for as long as the echo signals remained active, and because the action potentials generated by these signals controlled the muscles via the septum. But stretching the echo presented technical problems. For example, the delaying conductors would have to be twenty centimeters long to yield an echo lasting one second. The animals of that period were not that long overall. Could this even be the explanation for the giant size of some extremely primitive forms of life present in the ocean during the early stages of evolution? But the equation: Large Body = Longer Neuronal Delaying Conductors has a fatal flaw, because volume increases by the power of three in proportion to body length and higher water resistance causes an exponential increase in energy requirements that would sooner or later become insatiable.
So, was there some other way to enhance memory time and prevent the predator’s memory of the prey disappearing in a fraction of a second?
The solution was a technical trick – the output of the integration neuron was recycled as input. The olfactory receptors were excited when prey approached. The action potentials were transmitted from the cortex into the delaying conductor. This was tapped as often as possible by the echo neurons. The resulting echoes were transmitted to a common integration neuron along axon conductors that were better myelinated and therefore “faster”. Another new neuron – the recycling neuron – received these signals and routed them quickly along its myelinated axon to the starting point, i.e. to the granule cell of the olfactory cortex, thereby closing the circuit. Modern-day physicists call this recycling of input to point of origin ‘feedback’. This is the mode of action of modern electronic oscillating cycles.
Fig. 3: Use of feedback to produce continuous oscillation
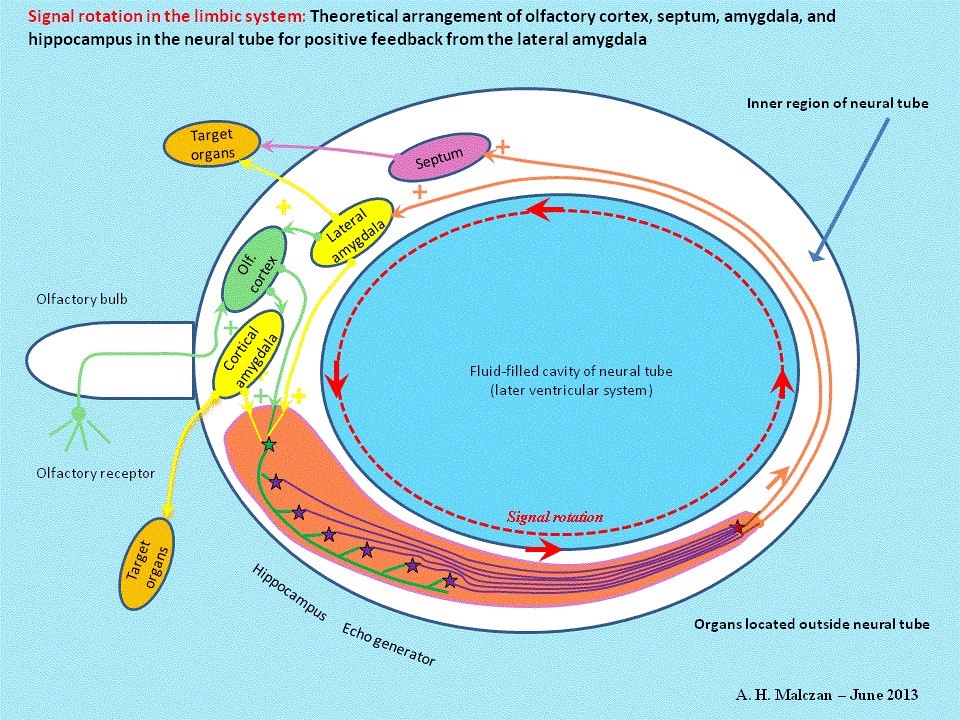
An oscillation is generated by excitatory, i.e. positive, feedback of output to input. This enables a single impulse from an input signal to generate a continuous oscillation. This type of oscillation signal is generated in the limbic system by positive feedback into the echo generator. The action potentials circulate continuously on axons forming a closed circuit.
Fig. 4: Continuous oscillation in limbic system:

The benefits obtainable from gigantism degenerated into impediments with the evolution of echo generator feedback. The olfactory memory became independent of body size, especially in insects, many of which gradually morphed from their once gigantic forms into smaller and even very tiny ones. Systems with feedback-capable echo generators no longer needed elongated delaying conductors. Dwarfish animal forms were equally capable of developing good olfactory memories. Millions of years later this sort of dwarf growth would prove remarkably beneficial in proto-mammalian life forms in an era when planet Earth was under violent bombardment from comets, its atmosphere darkened by dust, its vegetation withering away and food becoming scarcer and scarcer. Giant animal life forms like the dinosaurs were unable to cope with this catastrophe and became extinct. The smaller forms with feedback-capable echo generators survived.
Thus, the first evolutionary step was the transformation of one action potential triggered by an animal’s olfactory sensors into the series of forty action potentials used in our example by using echo neurons to tap the delaying conductor forty times. The next step was to plug these echo conductors together on a single integration neuron, on which the forty time-lagged action potentials were brought together in a time-lagged sequence. This yielded a highly desirable duplication of frequency that enhanced the system’s reactive sensitivity. The duplicated signals originally produced by the granule cells were routed back to them as input by a recycling neuron. To enable this, the delaying conductors, the echo conductors for the individual echoes, the output conductor for the secondary echo emitted by the integration neuron and the feedback conductor of the recycling neuron had to form some sort of circular structure, in which the end was in contact with the start. Many million years later, neurologists would name a structure of this kind in the limbic system the Papez Circuit. And they named the neurological structure of granule cells, delaying conductors, echo neurons and integration neurons the hippocampus.
The recycling neuron was now able to transmit its action potentials to two different points, either to the hippocampus or back to the olfactory cortex. Nature opted in favor of security and used both solutions. The result was that the recycling neuron routed the output signal back to both the olfactory cortex and the subiculum of the hippocampus, thereby closing the oscillation circuit at two points.
The recycling neuron linked to each delaying conductor was also able to transmit the output signal to other cerebral subsystems, for example, to the muscles, to the systems responsible for controlling blood pressure and to all the other functions enabling pursuit and capture of prey. The result was that the recycling neurons came together during the course of the evolutionary process to form a neuronal core emitting the output responsible for control of the key physical functions facilitating pursuit of prey, attack and defense and, of course, flight (in the sense of disengagement) – because the sense of smell not only helps to locate prey; it also warns of the presence of other dangerous predators capable of either competing for the prey or transforming a weaker predator into prey. We now call this neuronal core the amygdala. This lateral amygdala later acquired some important subsidiary cores that are now integrated into the amygdala and perform other functions. The section of the amygdala solely responsible for onward transmission of olfactory signals is now called the cortical core of the amygdala. As a host of other signals not originating in the olfactory system found their way to the hippocampus, the recycling neurons handling these signals formed the lateral amygdala. The evolution of the lateral amygdala is proof that the limbic system receives signals not originating in the in the olfactory system. Several researchers, including Pitkänen, Aggleton and Saunders, distinguish between the following substructures in the amygdala: anterior amygdaloid area, accessory basal nucleus, central nucleus, cortical nucleus (anterior and posterior parts), basal nucleus (magnocellular and parvicellular parts), lateral nucleus, periamygdaloid cortex, paralaminar part of basal nucleus. Part 4 of this monograph will show how the evolutionary process has pushed the amygdala’s original function of routing signals received from the hippocampus back to the hippocampus and the olfactory cortex as feedback into the background, in order to make way for a much more urgent development – digitalization of the analog output of the cortex layers. The lateral amygdala would have been quite adequate for feedback of the signals alone.
The lateral amygdala, which had originally been the core formed by the recycling neurons feeding signals originally emitted by the hippocampus back to the hippocampus, subsequently established contact not only with the sympathetic and parasympathetic nervous systems controlling physical activity, but also with the hypothalamus, that centrally-located cavity of gray matter responsible for suppressing pain during battle or flight, as well as the locus coeruleus and the tegmentum controlling alertness and reticular formation. Nature is a great believer in system theory.
Interlinked neurons form a chain transmitting the input signal. We could call this chain ‘signal-linked neurons’. Its axons and dendrites form a sort of data link, along which a given signal is transmitted. For example, a signal transmitted from a neuron in the olfactory cortex goes to the relevant granule cell in the hippocampus, then along the axon of the mossy fiber to the echo neurons, from there on to the integration neuron and then to the recycling neuron in the amygdala and so on. If one assumes that these linked neurons can not only exchange neuronal signals – which are known to be in the form of chemical substances, so-called transmitter molecules –, it is also reasonable to assume and has already been demonstrated that exchange of other substances, in particular marker substances, along the same chain is a feasible proposition. And if these marker substances are position markers for the intended target structures, all of the neurons in the chain will possess these target markers, which guide their axon collaterals to the locations specified by the position markers. The neurons of a chain of this kind are to a certain extent marker-related. This is understandable when they are all transmitting their output to the same target structures.
This would explain why, for example, not only the septal, but also the amygdaloid and the hippocampal neurons, and even the original neurons in the olfactory cortex have contact with, for example, the hypothalamus. All these signal-linked neurons form ‘tuples’ of marker-related neurons. And these then also have the same target regions. This strategy helps to circumvent problems arising from malfunction of one of the neurons in the chain. The target regions then receive the necessary input via the other marker-related neurons. This is the reason why both the olfactory cortex and also the septum, the amygdala and the hippocampus project in some cases into identical target structures.
The habenula already referred to above receives input from the septum and the amygdala, but also from the area olfactorius and the regio praeoptica, all of which interchange signals. This yields a greater degree of protection against malfunction and explains the remarkable robustness exhibited by neuronal systems.
On the other hand, it gives neurologists the problem of having to explain why each neuronal region is interlinked with most of the others for reasons that are mostly buried in Nature’s approach to system theory.
It should also be remembered that every single output neuron in the olfactory cortex has its own mossy fiber in the hippocampus, with its own echo neuron, Schaffer collaterals, integration neuron and recycling neuron in the amygdala. Also that the output neurons of the olfactory cortex represent different types of smell, each of which had its own oscillation cycle in the hippocampus where the signals are in constant circulation in order to prevent them from being forgotten.
To complete the picture, it should also be remembered that the olfactory cortex back in those days already had the capability to block neighboring receptors. Evolution had found it useful to hook up this blockade by installing interneuron links between every output neuron in the olfactory cortex and all the output neurons in their immediate vicinity. This enabled the stronger olfactory signals to suppress the weaker ones, thereby amplifying the stronger signals and attenuating the weaker ones. Insects already had this capability to block neighboring receptors in their olfactory systems. It was retained in many other neuronal cores of the cerebral system and amplified contrast in the output signals.
In principle, the primitive hippocampus described above generated for every single input signal (or more precisely for every cortical output neuron excited by an input signal) a continuous signal of higher frequency that kept on reminding the animal of the prey swimming past it and activating the physical motions enabling its pursuit. Similarly, the animal was prevented from forgetting the presence of an enemy by an oscillating signal triggered by hostile input. This mobilized and maintained for an adequate period the necessary action potentials for reactions to the enemy – either attack or flight. This was more or less equivalent to a memory. Once received, the oscillation triggered by the input signal circulated in the limbic loop and enabled the animal to remember the prey or the presence of an enemy. This memory function enabled pursuit of prey or, alternatively, flight or attack. The action potentials required for these reactions were supplied by the continuous circulating signals.
However, if, for example, each and every input action potential from the olfactory receptors generated forty new action potentials that were fed back to the granule cell at the start of the mossy fiber and each one of these was once again multiplied by forty and the process continued ad infinitum, wouldn’t this geometric progression produce a super-high-frequency oscillation of more or less infinite duration?
This is where the so-called ‘dead time’ of the neurons comes in. This was already an essential characteristic even at this early period of evolution. Every nerve cell took a very short break of roughly one millisecond after each action potential. This break is called the dead time. During this dead time the neuron is totally incapable of creating an action potential in response to even the most powerful excitation. This effectively put a ceiling on the firing rate. No neuron was capable of firing at a faster rate than that dictated by the reciprocal of the dead time. This is how Nature prevented a self-destructive increase in resonance frequency. It was Nature’s solution for providing positive signal feedback generating stable continuous oscillation without totally overloading the system.
The ceiling imposed on the firing rate by the dead time of the neurons lay somewhere within the range of 0 - 1000 Hz, i.e. approximately three powers of ten, and this created another problem. Signal strengths in Nature had in many cases much larger intervals than 0 - 1000. This made linear reproduction of signal strengths in firing rates by the receptors impossible. There was only one possible technical solution to this problem – logarithmic reproduction of natural signal strength in the firing rate. This is the reason why most receptors have a logarithmic curve. This keeps firing rates within the range dictated by the dead time. Only certain special neurons are capable of attaining firing rates of around 5000 Hz; most of them have firing rates significantly lower than 1000 Hz.
This maximum firing rate was now reached in the echo generator as well, as it fed back the output of the integration neurons as input into the granule cells. Unfortunately, this neurological solution had two serious design flaws. Firstly, it put a heavy load on the neurons involved because, once activated, they were compelled to keep on generating action potentials. There was a tetanic, i.e. higher-frequency, continuous oscillation in the circuit, in which the neurons continued firing without interruption. This was because each output from the hippocampus was recycled back to the hippocampus as input. These neurons fired at the maximum rate. This eventually led to overloading and premature death of the affected neurons, which consequently had to be replaced. It is now possible to demonstrate that the limbic system is still capable of producing new neurons and also new receptors.
The second flaw was much more serious. The rotation of the signals in the elementary Papez Circuit persisted not only for minutes, but also for hours and even days after the prey had managed to escape and was now miles away. The same applied when it had been caught, eaten and digested. Nature had to call a halt to this ongoing signal rotation, because it created the illusion that the prey was still around even after it had long since either fled or been eaten.
While it made sense to continue searching for a prey that had managed to find a temporary hiding place, if a further check of the immediate environment proved negative, it was reasonable to assume that it had escaped and was no longer in the vicinity. But the continuing rotation of the action potentials generated by the original olfactory fingerprint created the illusion that it was still somewhere close.
Although an illusion or mental image was basically a positive phenomenon, and could possibly be described as the birth of intelligence and consciousness, it was, in the context of search for prey, a dangerous illusion because it indicated the presence of prey that no longer existed.
Evolution had to supply a technical solution to both these problems, which will be designated here as hippocampus problems 1 and 2 respectively:
Hippocampus problem No. 1:
A way had to be found to interrupt the continuous rotation of the input signals with either short or longer breaks, during which the neurons could recuperate and, consequently, prolong their active life significantly.
Hippocampus problem No. 2:
A way had to be found to stop the continuous rotation of the input signals automatically after a certain time, when the intensity of the prey’s scent started to wane, either because it had escaped, already been eaten or the scent of new prey had made its appearance. There was also the possibility that other predators emitting a characteristic hostile scent could appear on the scene. In this case, the memory of the original prey had to be deleted and replaced by neuronal reactions enabling either flight, defense or attack.
The first solution was to make the continuously firing neurons gradually less sensitive to further excitation by the repeated input. The initial excitation of inactive neurons often produced a higher firing rate than subsequent excitations, i.e. the neurons’ sensitivity to repeat excitation declined with time. This kind of excitation curve can still be found in many of the receptors in the nervous system. But this solution was totally inadequate.
The solution to hippocampus problem No. 1 was provided by a neurological structure now called the septum. Septal cores likely existed prior to evolution of the hippocampus. These cores produce the transmitter substance acetylcholine, which has been responsible for activating not only muscles but also existing neurological structures ever since evolution of primitive animal life. The cholinergic activation system still performs an important function in the brain, even though it no longer directly controls activation of the motoneurons in the muscles. It therefore made good sense to use output from the hippocampus that had been triggered by scent of prey to activate the cholinergic system. Consequently, the excitation from the hippocampus projected into the septum, thereby enabling the animal to activate the necessary systems quickly when prey appeared or danger threatened.
The fact that cholinergic axons in the septum found the way back into the hippocampus may have been purely accidental. But excitation of the output neurons in the hippocampus was, in any case, of only limited benefit, because they were already excited. So evolution opted for another approach. Cholinergic axons from the septum excited the numerous GABAergic interneurons present in the hippocampus. Nearly every neuron core contains inhibitory neurons, whose principle function is to enhance contrast between output signals. This enabled the septum’s excitatory cholinergic output to reach the GABAergic interneurons in the hippocampus, because neurons automatically seek each other out.
Similarly, it made sense to give some of the GABAergic interneurons in the septum, those that were then and still are responsible for blockade of neighboring receptors and consequently contrast enhancement in the septum, the capability to form longer axons, because these could then form glutamatergic projection neurons in the septum, which would, in turn, block the glutamatergic projection neurons in the hippocampus.
We have decided to use the definition ‘direct blockade’ in this monograph to describe blockade of a core’s or a region’s output neurons by means of inhibitory projection onto those output neurons. Direct blockade acts directly at the signal’s point of origin, i.e. at the neuron whose output has to be suppressed.
Coining of the term ‘direct blockade’ automatically implies the existence of indirect blockade. We define indirect blockade as the excitation of neighboring inhibitory interneurons located in the vicinity of the relevant output neuron. As already also postulated, nearly all neuronal cores contain interneurons, most of which are GABAergic, whose main responsibility is to block weaker signals in order to enable stronger signals get through, i.e. they block neighboring receptors.
When projection neurons from other cores excite these inhibitory interneurons, the ultimate effect is blockade of a whole group of neighboring output neurons. We define this as indirect blockade. It enables the use of excitatory projection signals, whose inhibitory action stems from indirect excitation of inhibitory interneurons that, in turn, triggers blockade of the signal that would otherwise have been transmitted. It seems quite possible that these interneurons form a specific category responsible for switching from an excitatory to an inhibitory transmitter and no longer used for blockade of neighboring receptors.
When attempting to describe cytoarchitectural structures and linkages, it is always essential to analyze whether input into any region or core blocks the output-producing projection neurons located there or excites the inhibitory interneurons. The first case is direct blockade, the second indirect blockade. Use of these terms greatly simplifies description of inhibitory activity. But, as there is a third possible variant in the form of excitation of the projection neurons and also a fourth through blockade of the inhibitory interneurons, there are, in fact, at least four possible versions of neuronal contacts. This is the reason why identification of the neurotransmitter and the type of neurons contacted so important for analysis of neuronal circuits.
Thus, the hippocampus caused excitation of the septum and the septum had an inhibitory effect on the hippocampus, thereby using both direct blockade by GABAergic projection neurons and indirect blockade through excitation of inhibitory interneurons.
This demonstrates that the hippocampus and the septal cores already formed a closely cooperating unit back in the early days of evolution history. But why were these cores in the septum able to impose breaks that interrupted the higher-frequency continuous oscillation in the hippocampus? How was this possible?
According to one widely-held theory, the hippocampus and the septum were located at diametrically opposite points in the primeval brain, which still closely resembled the so-called neural tube. This meant that they were (by accident) at the maximum possible distance from each other. Consequently, the excitatory, glutamatergic signals emitted by the hippocampus had quite a long distance to cover before reaching the septum. And the return journey was just as long after they had been switched to GABA, an inhibitory transmitter, in the septum and the system’s topology routed them back to the hippocampus.
Neuronal signals were unable to follow a straight-line pathway through the neural tube, because this contained a fluid-filled cavity. Modern-day brains still have this cavity in a substantially modified form; it is filled with cerebrospinal fluid and is called the ventricular system. The body of the hippocampus still forms the border of the ventricular system in vertebrates and validates the neural tube theory. This border, which also contained nerve cells, had a curved surface that was the only available route for transmission of signals.
Assuming that the neural tube had a radius of around 10 millimeters and that the signals traveled in a semicircular curve from the hippocampus to the septum and took roughly the same route back, the total distance traveled corresponded to the circumference of the full circle. A circle with a 10 millimeter radius has a circumference of approximately 63 millimeters. An action potential traveling at a speed of 1m/s would cover this distance in 63 milliseconds. And this is the time that an action potential would take to travel from the hippocampus to the septum, be switched there to GABA and returned to the hippocampus. Thus, an active oscillation of 63 milliseconds’ duration was followed by an inhibitory GABAergic oscillation from the septum. And this totally suppressed the original signals from the hippocampus – for a period of exactly 63 milliseconds.
The original continuous oscillation was transformed by the strongly inhibitory feedback into a modulated, higher-frequency continuous oscillation with a time interval of 63 milliseconds. A tetanic excitation of 63 milliseconds’ duration is followed by signal-free interval also of 63 milliseconds’ duration. This would give the oscillation’s envelope curve an oscillation period of roughly 125 milliseconds, which would yield an envelope curve frequency of approximately 8 Hertz. All the foregoing numbers should be regarded as figurative.
This modulated oscillation of roughly 8 Hz in the hippocampus is nowadays called the hippocampal theta. It enabled primeval animals to remember an olfactory signal and to pursue prey that was trying to flee. Unlike an uninterrupted continuous oscillation, it does not overtax the neurons because they can take regular breaks of approximately 1/16th of a second, during which they can, for example, produce the necessary transmitter or freshen up their metabolism.
Fig. 5: The hippocampal theta of an active signal – a modulated titanic oscillation
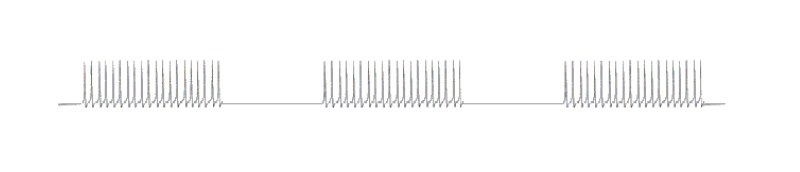
It should be noted that the magnocellular striosomal system operates (coincidentally) at roughly the same basic frequency. In this case too, it would be useful to examine the need to integrate suitably short intervals into continuous titanic oscillations (see Monograph Parts 1 and 2).
The interplay between the excitatory projection from the hippocampus to the septum and the inhibitory projection back from the septum to the hippocampus together with the time lag caused by the longer axon supplied the solution to hippocampus problem No. 1.
It is a well-known fact that Nature likes to provide fail-safe of backup systems. In the event of temporary or permanent failure of the direct GABAergic projection from the septum to the hippocampus this function could be taken over by an excitatory projection from the septum to the hippocampus. Cholinergic projection neurons in the septum could then excite the numerous GABAergic interneurons in the hippocampus and these would, in turn, inhibit the activity of the glutamatergic pyramidal cells in the hippocampus. This indirect variant is actually used in the mammalian brain to inhibit excitation in the hippocampus in addition to the one just described. .auto-style1 { margin-left: 0px; } .auto-style2 { margin-left: 5px; }
Fig. 6: Additional negative feedback from septum to hippocampus to induce intervals
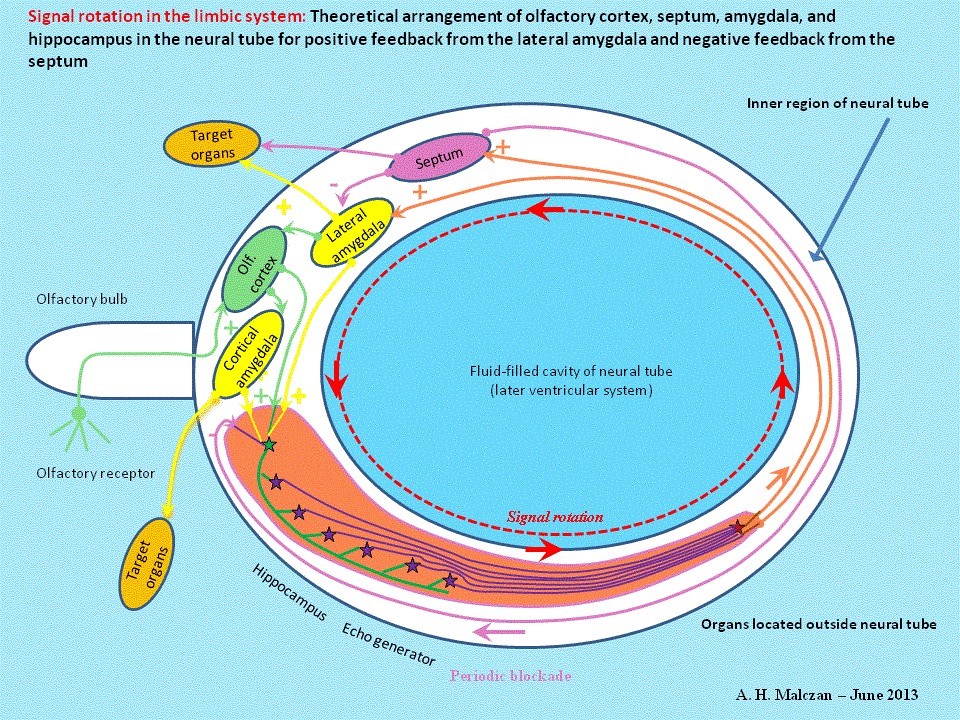
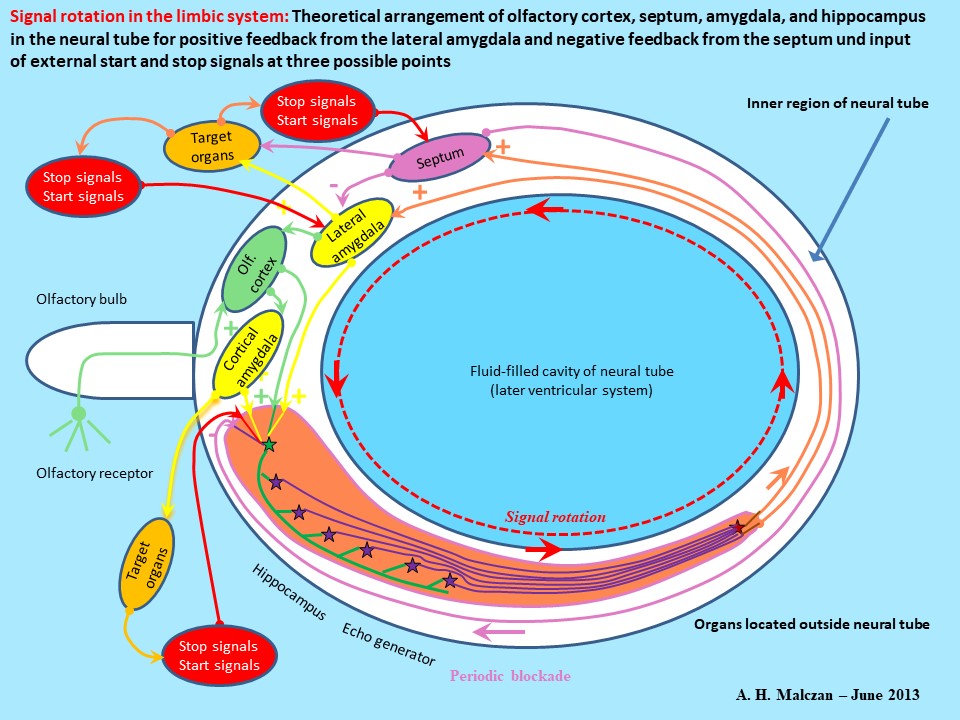
The availability of several fail-safe systems as backups to ensure correct functioning of neuronal circuits tends to complicate understanding of the basic scheme of operation. Direct and indirect blockade of the hippocampus by the septum ensures periodic interruption of oscillations. On the other hand, analogous GABAergic projections from the septum into the amygdala have also been observed. In the event of temporary dysfunction of the system for blocking the hippocampus, it seems possible that these projections would be able to take over the function of inserting short intervals into continuous oscillations of signals. Similarly, the excitatory projection from the hippocampus to the septum could likely be backed up by a glutamatergic projection from the amygdala to the septum. But these are not, in fact, observed for the simple reason that the amygdala and the septum are located too close to each other. Signals from the amygdala would reach the septum immediately. An excitation triggered by the amygdala would be followed almost instantaneously by a reaction from the septum blocking the amygdala, and this would seriously interfere with generation of the desired titanic excitation. Consequently, there is only the septum‟s inhibitory action on the amygdala. Thus, there are several fail-safe options available for provision of positive and negative feedback in the limbic oscillation circuit. A wide variety of phenomena can be observed in oscillation circuits. One interesting phenomenon seen in neuronal oscillation circuits with strong frequency multiplication and positive and negative feedback is standardization of signals. The strong multipication induced by the large number of echo neurons linked to one mossy fiber generates a tetanic oscillation with an almost constant firing rate dictated solely by the dead time of the neurons. As the integration neurons of these subsystems all have roughly the same parameters, they also have roughly the same dead time. The result is that signal activity in these subsystems produces tetanic oscillation of roughly the same frequency in each signal loop. On the other hand, negative feedback inserts intervals in the tetanic oscillation. And, as the time lags in transmission of action potentials from the hippocampus to the septum and back are roughly the same for all the oscillation circuits, the duration of the interval induced by septal blockade is also roughly the same. The result is standardization of not only the frequency of the titanic oscillation but also the duration of the interval. In addition, the various oscillations are not only standardized; they are also loosely linked to each other, i.e. partly synchronized. This is attributable to blockade of neighboring receptors by the numerous inhibitory interneurons. This standardization invalidates a whole lot of longstanding assumptions. We normally assume that the firing rate for a signal would increase with signal intensity. This is no longer the case in limbic oscillation circuits. During its passage through the mossy fiber a weak olfactory signal with a low firing rate undergoes an increase in frequency up to maximum level, which is determined solely by the neuron dead time. In a properly functioning hippocampus there are hardly any frequency differences between weak, moderately strong and strong olfactory signals. They are all raised to maximum frequency, and intervals are only induced by negative feedback and are of standardized length. This is the reason why Nature had to find ways and means to code varying strengths of olfactory and other limbic signals differently. The solution was digitalization of the signals. This made it possible to generate action potentials for signals of varying intensities, but the same scent on different binary conductors, because each signal strength used its own conductor. Plus, all active signals could use the same firing rate and were consequently standardized within the system. This type of signal standardization is now used in computers. It is the address of the conductor, not the bit signals, all of which are identical anyway, that determines the bit‟s importance. Digital computers also use active and inactive conductors, and not signal strength, in the same way. Part 4 of this monograph – to be published as a separate work – will explain how the formation of inhibitory interneurons in neuronal cores laid the key foundation stone for this digitalization. The GABAergic interneurons in the hippocampus also provide the solution to hippocampus problem No. 2. When the prey had been eaten, the digestion process started in the stomach. However, the ensuing increase in gastric activity was not only chemical. The digestive Page 15 system‟s receptors acted as sensors measuring the rise in chemical activity and converting it into neuronal activity. When digestive activity caused digestive system receptors to transmit stronger excitatory signals to the GABAergic interneurons in the hippocampus. This caused inhibition of the hippocampal theta of the olfactory signals and the relevant oscillations subsided. The activity in the digestive system exerted an inhibitory effect on the hippocampus and deleted the olfactory signals spinning around and oscillating in the loop. The animal had satisfied its appetite, forgot the prey and became drowsy. The same process occurs right down to the present day. Over the course of time, some other strong signals emitted by the “cerebral system” found their way to the inhibitory interneurons in the hippocampus. The result was that the hippocampal theta, which had formerly been an “eternal” value, was brought to a standstill by GABAergic blockade within minutes or, at most, hours. Another aspect of GABAergic blockade by interneurons is that each inhibitory interneuron reaches quite a large number of hippocampal granule cells. This means that a large number of neighboring mossy fibers are also synchronously inhibited, which, in turn, synchronizes oscillation of the hippocampal neurons. It is this phenomenon that drew attention to the changes in levels of the hippocampal theta. The more neurons firing synchronously in the hippocampus, the better are we able to detect, for example, the theta waves in an EEG through the scalp. There is an analogy here in the magnocellular striosomal system, where the numerous oscillations in the elementary oscillation circuits are (loosely) synchronized by dendrodendritic linkage with the dopaminergic neurons in the scattered cells of the substantia nigra pars compacta (see Parts 1 and 2 of this monograph). External excitation of the GABAergic interneurons in the hippocampus enabled interruption of signal oscillation in the hippocampus either after capture or escape of the prey or if new signals warned of presence of an enemy. But the system formed by hippocampus, septum and amygdala now had three possible points of input entry. There were two other ways to interrupt oscillation of any specific group of signals. Blockade of the glutamatergic recycling neurons in the lateral amygdala was one alternative to excitation of the GABAergic interneurons in the hippocampus. But this blockade had to last as long as the cycle time of the signals, otherwise all the individual echoes would not be deleted. During the course of evolution the neurons activating this blockade formed their own neuronal core, which is now the central core of the amygdala and has an inhibitory projection into the lateral amygdala enabling it to completely interrupt oscillation of relevant signals. The key characteristic of the central amygdala is its GABAergic neurons, which project with an inhibitory effect into the neighboring cores of the amygdala. Part 4 of this monograph will explain how these neurons were able to take on a much more important role that enabled vertebrates to take a massive leap forward in intelligence, namely, digital signal processing and the genesis of learning ability. This will not be discussed here because this section focuses on examination of signal processing within the limbic system. The point to be noted here is that the central amygdala was able to switch incoming excitatory signals into the inhibitory transmitter and transmit stop signals to the lateral amygdala. A second alternative way to interrupt specific signals was by strong and sustained excitation of the GABAergic projection neuron in the septum receiving the pooled echo signals from the integration neuron. This triggered an inhibitory GABAergic signal to the pyramidal cells suppressing their activity. The longer-lasting excitation of the GABAergic septal neurons caused similarly prolonged GABAergic blockade of the relevant hippocampal neurons and interruption of the signal‟s rotation. Thirdly, there was the alternative pathway via the septum already referred to. The hippocampus contains a large number of inhibitory interneurons using GABA as the transmitter. Their role is to enhance output contrast and suppress weaker signals by blockade of neighboring receptors. It would therefore be possible (as already described) for cholinergic projection neurons in the septum to induce prolonged excitation of the GABAergic interneurons in the hippocampus. This would also cause blockade of signal oscillation. If the Page 16 cholinergic septal neurons were subjected to prolonged external excitation, this would cause collapse of the relevant signal oscillation in the hippocampus. Either the GABAergic projections in the hippocampus or the lateral amygdala could bring signal rotation to a standstill by direct blockade, or excitation of the GABAergic interneurons in the hippocampus or the lateral amygdala could terminate rotation of specific signals. The same effect could be achieved by exciting the relevant neurons in the central amygdala. But, if the prey managed to escape, no digestion could take place. No signals would be emitted by the digestive organs to excite the interneurons in the hippocampus or the amygdala and oscillation of the signals from the prey could not be eliminated via this pathway. Consequently, it was essential to introduce an internal timer system that would – after a given period of time, i.e. the maximum duration of pursuit of prey – be responsible for interrupting signal oscillation. In the early days this will definitely not have been a 24-hour circadian rhythm from the nucleus suprachiasmaticus, but there is certain to have been a neuronal core with a rhythm spread across, for example, a period of perhaps two hours. If activity of the neurons in this core spiked to a maximum every two hours, their action potentials would be able to reach the GABAergic interneurons of the hippocampus and delete all signals rotating there. Alternatively, the GABAergic projection neurons in the septum could be excited for a prolonged period to induce blockade of the pyramidal cells in the hippocampus. The timer would also be able to interrupt signal rotation by either direct or indirect blockade of the amygdala. Any animal not possessing a timer of this kind would waste time and energy. It would continue pursuing the scent of a prey for days, weeks or years, perhaps even for the remainder of its life, even though the prey had long since gone elsewhere. The signals spinning in its hippocampus and indicating the presence of prey would be an ongoing illusion. Senseless pursuit of this illusion would waste increasing amounts of energy that could quickly lead to the animal‟s death from hunger, unless another potential prey emitting a stronger scent happened to appear on the scene. But an animal periodically, i.e. every two hours, deleting the signals spinning around its limbic system would be able to rest while awaiting the arrival of juicy new prey, whose scent would trigger oscillation of a new signal that would activate the physical systems enabling pursuit and attack. Modern-day animals have in-built time rhythms with cycles lasting from a few hours to a full day. Interference with this periodic deletion of signals, e.g. by denial of sleep, causes serious problems. Life in general needs to follow a cyclic pattern. Capture of prey, consumption of prey, digestion of prey and resting follow a set cycle. And the fact that the highly sensitive sense of smell existed at all created a need for specific organs capable of supporting the phases of this cycle. For example, any creature continuously disposing of the waste residues arising from metabolization of its food into the waters of the ocean left a strong scent leading directly to it. But a creature developing suitable cavities like a bladder or a rectum for temporary storage of these metabolites was able to stay hidden longer from the olfactory antennas of its predatory enemies by waiting until it found a safe place to evacuate these cavities. The system‟s basic mode of function involving feedback to the hippocampus favored a cyclic life pattern. Initiation of both olfactory and cyclic signals was by timer. These signals gave rise to sustained reactions that could only be inactivated by stop signals. Where necessary, the timer was made responsible for this periodic inactivation as well. There was another reason why periodic deletion of limbic signals was essential; each rotating signal produced new action potentials that activated reactions. If an action potential from the olfactory cortex entered a mossy fiber, the tapping of this mossy fiber caused the CA3 pyramidal cells to generate a whole series of new action potentials. When these reached the amygdala, the septum and the corpus mamillare and excited the neurons there, these organs responded with still more action potentials. Thus, once in oscillation, a signal could only be stopped by receptor blockade from a new, neighboring signal, because rotation fatigue in older signals led to a relative decrease in firing rate. The only other option was periodic deletion by a suitable timer. These could, of course, be programmed to specialize in selected groups of signals. For example, some timers could perhaps have been responsible only for creation of hunger sensation by deleting earlier signals of satiety from the digestive system. Page 17 This deletion was a must, otherwise the creature would eventually die of hunger, still firmly believing the message from its limbic system confirming that it was replete. The behavioral studies in rats, where the animals developed a preference for self-actuated stimulus of their limbic system over the opportunity to eat, demonstrates the dangers inherent in failure to “reset” the limbic system and delete circulating elementary limbic stimuli. This type of RESET command to the limbic system, e.g. during sleep, also generates greater readiness to receive the next day‟s new signals. On awaking, the head has been emptied. Any creature unable to do this will inevitably waste strength (time?) and energy processing signals that are purely imaginary and no longer valid. Many of the signals spinning around the limbic loop and now scheduled for periodic deletion, especially the new ones containing formerly unknown information, will no doubt have been useful information and worth memorizing. Evolution reacted to this by developing algorithms for transfer of these temporary signals into the cerebellum for permanent storage. This occurs during the REM phases of sleep. This required availability of technical systems enabling access of limbic signals to the cerebellum. Another essential requirement had already been fulfilled - LTP and LTD in the cerebellum were already possible, because the limbic signals were already available in the form of tetanic excitations complete with the necessary intermittent control intervals. During these intervals the Purkinje‟s cells responsible for the storage tested the results of the preceding synaptic modification of linkage strength in their own and other signal detectors (see Parts 1 and 2 of this monograph). In this way, the signals were transferred from the active limbic memory into the passive cerebellar memory. In summary, it is clear that the neurons responsible for periodic interruption of oscillation in the so-called theta rhythm, are equally capable of terminating signal oscillation definitively. This is possible if they are they are excited long enough to ensure that every one of the circulating action potentials has been deleted by blockade. But it is not only the signals from the escaped prey that need to be deleted promptly. Prey that was captured and eaten also triggered corresponding signals from the taste receptors during the eating process. These also found their way to the hippocampus, from where they were relayed to the stomach to trigger, for example, hydrochloric acid production for the digestive process. This activation of the digestive system and the signals triggering it and still circulating in the limbic loop were no longer required when digestion was completed and could even be injurious if not deleted. This created a need for monitor receptors capable of predicting completion of the digestive process and deleting the relevant signals in the limbic system. Thus, there was obviously a large number of reasons for deleting rotating signals. But reversal was also possible. Triggering of signal rotation by external stimuli also proved extremely useful. An inactive and totally new signal, which did not necessarily have to be olfactory (e.g. hunger sensation), from other areas could be input into the hippocampus, e.g. by transmission via excitatory neurons into the lateral amygdala. This type of neuron already existed. It was possible to transmit the excitatory input to the recycling neuron in the lateral amygdala that was specifically designed for this purpose. The excitation caused by this neuron initiated oscillation of this specific signal in the limbic circuit. It was also possible to subject either an integration neuron (CA1 pyramidal cell) or an echo neuron (CA3 neuron) to direct excitation in the hippocampus. A third potential option was to excite a cholinergic neuron in the septum, which – contrary to the concept discussed so far – would, in turn, excite either the granule cells or the pyramidal cells in the hippocampus. It is up to the neurologists to investigate whether this pathway was, in fact, used. It would be perfectly feasible in purely theoretical terms, but would require evolution of a new category of neuron that was not available in the original concept. This would introduce an ambiguity into the system that would constitute a nuisance factor. The key point to remember is that there were several available options for both initiation and termination of a signal oscillation in the limbic system.
Skizze 7: External initiation of an inactive signal and external termination of an active signal
One of the main functions of the oscillating signal was to keep the memory of the initial input signal alive. The memory of the prey or the danger was preserved for a longer period. During its existence the action potentials from this signal activated the relevant muscles and other physical systems enabling correct reaction to the situation. The signal was present in a type of oscillatory form and was consequently stored, but not statically stored. It was not stored in cerebellar storage cell, as described in Part 2 of this monograph. Storage in the limbic system was dynamic in the form of an oscillation, during which the signal was present and active, as though the stimulus that had triggered it, e.g. characteristic scent of the prey, was still present. In the meantime the actual original scent had frequently faded or even disappeared altogether, and the oscillating signal was merely a memory creating the illusion that the scent was still present.
Even today, a memory is still only a mental concept and therefore, in the final analysis, an illusion. But without this type of illusion the past would no longer exist; there would only be the extremely short present of the immediate moment. In this respect, there is a close relationship between memory and illusion.
Signal oscillation in the limbic system serves the purpose of temporary or preliminary storage. The nearest analogy to this in the computer world is the use of interim stack storage, from which data is retrievable when needed. The limbic system is a neuronal stack memory of the same kind, in which data are stored temporarily in the form of rotations around the various circuits until they are needed again. The oscillations generated by these rotations make them perceptible. An active signal is present in the limbic system in a form defined in this monograph as an oscillation. Whereas present-day computers simply have memories, neuronal systems have two distinct types of memory – the active memory and the passive memory. Storage of a neuronal signal in an active memory generates ongoing computer output. The storage neurons generate action potentials continuously. This constant output only ceases when signal rotation is completely deleted. A pendulum as an example of this. The signal remains active as long as the pendulum is swinging. When the pendulum stops or is stopped, its signal becomes inactive.
In contrast, passive memories emit no output at all when a signal is stored in them. Although the strength of the synapses containing the data is modified at time of input, their neurons deliver no output whatsoever. This type of memory is present in the cerebellum. A passive memory delivers output in one case only – when it receives input that is either identical or very similar to the signal stored in it. Then, and only then, does a passive neuronal memory deliver output.
A passive neuronal memory compares the incoming input with the relevant stored data. If these are identical or very similar, it will deliver an output signal for as long as an adequate degree of similarity between input and stored signal persists. This is roughly how passive electronic computer memories function.
This would perhaps be a convenient moment to provide some explanations of the way in which computer memories work, as experience shows that misconceptions exist in this area.
It would be misleading to compare a computer memory with a cabinet full of drawers containing a selection of articles, e.g. screws. Comparison with a full football stadium or a busy DIY store would be nearer the mark. There is a loudspeaker announcement: “The parents of Johnny Doe from Philadelphia are requested to come to the entrance to pick up their 5-yearold son.” Daddy Doe stands up and runs to the entrance where his son is waiting.
This is roughly how a computer memory works. An address signal (like the announcement stating the name) is input and travels through the address conductors to the memory cells. And the memory cell bearing the same address as the one transmitted via the conductors delivers its content onto the data conductor. The user needs to know which memory cell contains the content he wants to retrieve and specify this in the input inquiry. The computer understands the inquiry as meaning: “What does the memory cell with the address x contain?”
Present-day (year 2013) computers only have passive memories. Active memories comparable to the limbic system have not yet made their appearance in the computer world.
The fact that active and passive neuronal memories exist has consequences for our consciousness. The multimodal image of the world contained in our brain is produced by the active memory. Our perceptions, sensations, imaginations, ideas and feelings are represented by action potentials spinning around and oscillating in limbic circuits and creating signals that interact with each other.
In contrast, our life story is stored in the passive memory of the cerebellum. If this were to be permanently active, we would constantly be watching a film of our past life. This would inevitably have a seriously disruptive effect on our normal everyday life. This is the reason why memories not at present needed are transferred to passive storage. As already explained in Part 2 of this monograph, specific portions of these inactive data are reactivated when the inverse cerebellum converts these data into oscillations and outputs these to the active memory. It is only when the data have been converted into oscillations that we once again become capable of generating ‘tangible’ memories. A good example of this is the anecdote of the bricklayer’s accident already discussed in Part 2 of this monograph. A bricklayer falls off a high building. As he plummets to almost certain death, his past life flashes past him in countless vivid mind images. A lucky chance saves his life and he is able to tell people about this experience. What had happened was that his inverse cerebellum, activated by the stress situation, delivered these images to the thalamus, which is incapable of distinguishing between external and self-generated input. These signals gained access to the limbic system in oscillation form and became tangible memories.
As demonstrated in Part 2 of this monograph, active signals in the non-limbic system can also be present in oscillation form, if this is enabled by blockade of neighboring receptors (Malczan’s Oscillation Theorem).
In addition to the aspects already mentioned, another effect was caused by blockade of receptors of the pyramidal cells in the hippocampus. If every hippocampal output neuron were to occupy its own GABAergic interneuron and excite this exclusively with its own output, this interneuron would, when excited, blockade all output neurons within its reach in a fairly large catchment area. In this monograph we use the definition “blockade of neighboring receptors” to describe the process by which output neurons inactivate each other reciprocally inhibition of their receptors. This phenomenon ensures that, from the mass of signals input into the system, only the stronger ones prevail. The weaker signals circulating in the vicinity are either partially or completely suppressed by this blockade of neighboring receptors.
It seems possible that malfunction was involved in the present case. If GABA blockade of neighboring receptors does not occur, the hippocampal system is no longer able to continue inserting short breaks and suppress the weaker signals. This triggers an avalanche of activity resulting in the condition known as epilepsy. Thus, it seems possible that epileptic fits are caused by a disruption of circuits following failure of the interneurons in the hippocampus to perform their GABA blockade function. Failure of the GABAergic septal neurons had the same effect. The periodic interruption of oscillations in the hippocampus ceased and the system started to oscillate at highest frequency without any breaks, i.e. another epileptic fit. The reason why severe epileptic fits are so serious is that a hyperactive hippocampus causes extreme activation of the septum and the amygdala, and this in turn leads to total activation of all body systems.
This explains why there is the alternative of an inhibitory GABAergic projection from the septum to the amygdala. This enables the amygdala itself to initiate periodic interruption of an oscillation when temporary malfunction of the hippocampus is threatening to make it continuous. This ensured that a fail-safe function was in place, but made it considerably more difficult to understand how it functions.
One special feature of this blockade of neighboring receptors has already been mentioned in Part 2 of this monograph; it enabled the evolution of intelligence and meditation. Blockade of neighboring receptors has a limited radius of action. A GABAergic interneuron can only inhibit an output neuron whose dendritic process is within reach. The area of receptor blockade if finite and usually small in diameter. This is of relevance for the signals in the higher association areas, most of which are spatially scattered. The Potentiation Theorem (see Part 2 of monograph) postulates that the number of signals in a complex increases, and the spatial distance of the neurons in a higher complex tends to increase statistically with the degree of abstraction. Consequently, if signals from a complex in the higher cerebral levels find their way into the hippocampus, they will tend to rotate within the hippocampal system for a significantly longer time – perhaps hours or even days. The more abstract the signal, the longer will it continue rotating in the hippocampal system, because the probability that other similar and stronger signals will arise is statistically low and, consequently, blockade of neighboring receptors declines to a low level.
The question whether signals of this kind from higher complexes reach the hippocampus will be answered later.
Rhythmic tetanic oscillation is an extremely important factor in the hippocampus-septum-amygdala system and we will refer to it in future as the limbic system rhythm. The term “hippocampal theta” is also used.
The existence of periodic high-frequency oscillation paved the way for evolution of another innovation. Neurologists like to describe high-frequency oscillations as tetanic excitation. But tetanic excitation is an essential prerequisite for development of LTP and LTD.
Without long-term potentiation and long-term depression it is impossible for a long-term memory to develop. It has already been demonstrated in Parts 1 and 2 of this monograph how LTP and LTD in the cerebellum lead to learning processes. This monograph still has to show that LTP and LTD are used in the limbic system.
It has already been explained how signals – both olfactory and other types – can be stored preliminarily, i.e. temporarily, in the limbic system in the form of signal rotation. It still needs to be explained how these signals can be transferred to a permanent form of storage. This will be done in Part 4 of this monograph, where it will be explained how the cerebellum commits new limbic signals to memory.
At the end of this excursion into epochs long past we intend to make some suggestions as to how the Papez Circuit evolved. The signals emitted by the hippocampal oscillation system prolonging the memory of prey or warning of the presence of other predators were needed for further processing in the nervous system. On the one hand, the septal system was activated; on the other, output from the lateral amygdala triggered a strong system reaction when attack or flight became necessary, while the central amygdala was responsible for weakening excitation by blockade of the rotating signals.
This limbic system was the first temporary memory in the cerebral system. Its signal oscillation process was so successful that all neuronal signals gradually found their way into the limbic system.
The output from these subsystems, especially from the hippocampus, was collected in a newly-evolving switching core, the corpus mamillare and proceeded from there to the nucleus anterior thalami, a newly-evolved core in the thalamus specifically designed to handle limbic signals. This projected into a new cortical structure, the gyrus cinguli. This structure is a cortical area ,over which the new cortex of modern vertebrates was later superimposed to form the inner gyrus in the brains of modern-day vertebrates. This closes the circuit, because it is the gyrus cinguli which transmits most of its output to the hippocampus. This small inner limbic oscillation circuit, comprising hippocampus, septum and lateral amygdala, in which signals are temporarily stored by rotation and signal oscillation, acquired as a result of this redesign an additional outer oscillation circuit. Neurologists now call this outer limbic oscillation circuit, consisting of a cyclic linkup of hippocampus, anterior thalamus and gyrus cinguli the Papez Circuit. In the opinion of the author of this monograph, its principal theoretical role as a system responsible for handling information is temporary storage of limbic signals in the form of oscillations in closed circuits, in order to prevent these signals from disappearing into oblivion.
While the inner limbic oscillation circuit mostly handles olfactory signals and signals from the autonomic nervous system, the outer circuit handles signals of a non-olfactory nature that have found their way into the limbic system. Many of the latter are so-called unmarked signals. These are the signals that the cerebellum has not yet been able to commit to memory completely. After being marked in the cerebellum, signals of this kind oscillate between the direct and the indirect cerebellum (see Malczan’s Oscillation Theorem – Part 2 of this monograph) to reach the central amygdala and from there the lateral amygdala, where they delete the original signals still spinning around the limbic memory in temporary storage. Thus, the only signals rotating in the limbic system are unmarked, active ones not yet committed to memory in the cerebellum. More precisely, they are in all cases the new signals received by the creature. It is for this reason that we always associate the hippocampus and the amygdala with new signals, because it is always in the non-limbic system that signals already committed to memory have their activity phases. Loss of limbic system function equates with loss of learning capability. This is because new signals first have to stay spinning around the limbic loop until they have been committed to memory by recruitment of the neurons required for storage of memorized data in the cerebellar system. These are obtained from the available stock of proneurons. And this process of transformation of proneurons and formation of axons to link up the resulting neurons (i.e. learning) can last hours or even days. If it proves impossible, the creature loses its learning capability.
Signal oscillation – which is also observed in the non-limbic system (see Malczan’s Oscillation Theorem – Part 2 of this monograph) is a phenomenon that should be examined in an attitude totally devoid of emotion. Although we always associate limbic signals with emotions and feelings, this is because of the significance of the signals that have to be assessed – like, for example, those from the amygdala for a decision to either fight or run away and fight another day. The context-related and emotional significance of the limbic system’s signals must not be allowed to distract attention from the mission actually entrusted to the limbic system by evolution – the preservation of important signals for a sufficiently long period. The limbic system created the first-ever memory based on neuronal circuitry in the history of evolution.
The concept of temporary storage of a signal form by oscillation in a closed loop was so successful that it was also used in the non-limbic system during the subsequent course of evolution, where it was responsible for the genesis of intelligence and meditation, as already demonstrated in Part 2 of this monograph.
It is now therefore time for a meticulous test of this theory. A test of the theory by the author himself revealed some flaws that will have to be corrected (in Parts 4 and 5 of this monograph). These relate, in particular, to the double and quadruple negation in the basal ganglia system. We can anticipate some new, corrected insights in this area that will help to clear the way for better understanding of the brain’s digital functioning.
In summary, it can be concluded that use of the limbic system and the signal rotation procedure made it essential for all active signals to be interruptible. Hunger created an urge to hunt for prey. A full stomach canceled that urge. Pairs of signals were also formed, in which the two parts were diametrically opposed or, perhaps, complementary to each other – hunger/satiety, desire/aversion, tasty/disgusting, warm/cold, attack/flight, courage/cowardice, love/hate, every limbic signal had an inverse signal representing the other extreme. And all these paired signals could only be stopped by activating the other extreme. Thus, in all pairs of this kind, one of the extremes was always active, and the other inactive. The system could be described as dual or bivalent. And because there were so many of these dual of complementary signals, animals lived in a multidimensional dual world. We therefore interpret the limbic system itself as a multimodal assessment system. And because many limbic signals are extremely complex and contain a mass of sub-elements, we frequently interpret them as emotions. The elementary signals concealed within them are too complex for us to understand. But the fact remains that every emotion in the limbic system has its opposite emotion and only one of these can be active at any time.
It got really complicated when it became possible to gauge the strength of these emotions, because at some point in evolution the limbic system acquired the ability to digitalize signals. Suddenly, nothing was simply black or white anymore, there was a whole selection of different shades of gray in between. This may sound illogical. But can you always answer questions like “Are you warm enough?”, “Are you hungry?”, “Do you feel well?” with a clear yes or no. There can always be intermediate nuances like: “I feel very warm”, “I’m quite warm”, “I feel a bit cold.” We all know the statement used by the weathermen: “There is a 45% probability of rain”. Percentages are classic examples of intermediate assessments. The arrival of interim values in the limbic system’s formerly dual raster made the world multidimensional and its apparent continuity questionable, because a finite number of neurons can only produce a finite number of interpolations in the form of intermediate values. The apparent continuity of the world and its parameters is a neuronal illusion of the mind perceiving it. Although the world is mostly continuous (unless you are looking at it through the eyes of a quantum physicist), the fact that the brain contains only a finite number of neurons means that it can only be depicted discontinuously in digitalized form. The still elusive secret of how continuous signals from the environment are digitalized in the brain will be reviewed for the first time in Part 4 of this monograph.
Before we start to summarize the insights gained so far, there is one simple question about the limbic system that needs answering: Approximately how many different limbic signals are present in a human being?
If we assume that most of these signals, when active, are spinning around the outer limbic oscillation circuit – the Papez Circuit –, the answer to that question has been lying around for quite a time. Each one of these signals has a projection neuron in the corpus mamillare. As far back as 1957, Powell, Guillery and Cowan calculated that the nucleus medialis of the human corpus mamillare contains some 400000 nerve cells. That figure is stated on page 147 and in Table 163 on page 333 of Blinkow &Glezer’s up-to-date work The Central Nervous System in Figures and Tables. This means that a human being can learn and distinguish between roughly 400000 different limbic signals. Whereby the limbic system is present in duplicate
ISBN
978-3-00-045141-6
Monograph von Dr. rer. nat. Andreas Heinrich Malczan