Theory of the neuronal circuity of the brain and analytical thinking
ISBN
978-3-00-037458-6
ISBN 978-3-00-042153-2
Monograph of Dr. rer. nat. Andreas Heinrich Malczan
Part 2.9 The substitution of the magnocellular climbing fibre signal by the parvocellular
So far, we have put a lot of effort into explaining how a single primary climbing fiber signal, which was generated from the activity neuron of a cortex cluster by averaging over the signal neurons of the cortex cluster, eventually resulted in many hundreds or thousands of secondary climbing fiber signals.
In the simplest case, the primary climbing fiber signal was distributed to the many secondary climbing fiber axons. An evolutionary first variant was the central distribution via a common distribution neuron in the nucleus olivaris.
More advanced was the sequential distribution of the primary climbing fiber signal over a sequential distribution chain. You'd think now nature would have reached its goal. Were it not for the differences in learning ability between the species. And were it not for the unsolved problem of the development of intelligence in this monograph.
We have recognized that the Cerebellum is forcibly imprinted. Embossing is achieved by activating the climbing fibre signal once in a free Purkinj group for the duration of the embossing period. But this is the end of the learning process. The theory so far only shows how a signal occurring for the first time in the cerebellum can be learned in a very short imprinting period of about one second. But besides relearning there is also the modification and adaptation of the already learned signals to new conditions. Therefore, there had to be a neural circuit that produced exactly these abilities. It was the mathematicians' prerogative to recognize these abilities, because the associated theory is a mathematical one. The theory of neural networks provided the algorithm by which intelligence can arise. It is based on a long learning process. Here we have a temporary contradiction to the theory presented so far.
Nature must have found a way to modify knowledge already learned. And here lies the key to the new way, which obviously had to exist.
No living being learns anything within a second and keeps this knowledge forever and ever. The exact opposite is true. Acquired knowledge is constantly re-layered, re-evaluated, updated. So how is this knowledge, which is already burned into the Purkinje cells, modified afterwards? How does the constant learning process proceed?
How is the re-imprinting of the Purkinje cells carried out?
If a strong climbing fibre signal caused the "first imprint", how could a "new" climbing fibre signal cause a "re-imprint", in which the knowledge is updated.
Example:
- First learning process: dogs eat bones.
- Second learning process: Wolves eat bones.
- Third learning process: Lions eat bones.
- and so on
- and so on
- Last learning process: Jackals eat bones.
If the first learning process at Fritzchen Müller took place on 01 March 2011, how can the second learning process take place on 30 May 2011? And like the other learning processes, if between each of them days, weeks, months or years pass.
The Purkinje cell with the content "eats bones" must react to the input "dogs", later additionally to the input "wolves", and finally also to the input "jackals".
With each learning process, new moss fibre signals are added as characteristic signal components. How is this handled in Cerebellum.
First of all, from the author's point of view it seems urgently necessary to define some terms in order to shorten future texts and to create a certain system-theoretical order. Neurologists will already know these terms. Here, however, they may be clarified.
Definition 2.7: Primary cortex, primary cortex clusters
The cortex areas that receive their input from the receptors of the nervous system are called primary cortex areas. The associated cortex clusters are called primary cortex clusters.
Definition 2.8: Primary (parvocellular) thalamus
The thalamus that supplies the primary cortex with the receptor signals is called the primary (parvocellular) thalamus.
Since the primary cortex can be divided into clusters, we can also define thalamic clusters by "tracing" the input in the primary thalamus.
We use the parvocellular specification for the primary thalamus because there is still a primary thalamus that sends the magnocellular signals to the cortex. This is, for example, the nucleus centromedianus thalami, possibly this also applies to the intralaminar nuclei. So where confusion is possible, the addition parvocellular should be used.
Definition 2.9: Primary cerebellum, primary cerebellum clusters
The signal neurons of the primary cortex clusters project via the bridge nuclei into the moss fibres and nuclear neurons of a cerebellum cluster. This cerebellum cluster also receives the climbing fibre input derived from the basal ganglion system of the same cortex cluster from the activity neuron of this cortex cluster. It is irrelevant whether the secondary magnocellular climbing fibre signals were generated by central or sequential distribution. We refer to this cerebellum cluster as the primary cerebellum cluster.
In the primary cerebellum cluster the complex signals are formed, which are composed of the elementary signals of the primary cortex cluster.
The output of the primary cerebellum cluster reaches the thalamus via the positive nuclear neurons. This thalamus region also receives a name.
Definition 2.10: Secondary (parvocellular) thalamus
The output of the positive nuclear neurons of the primary cerebellum cluster reaches the secondary (parvocellular) thalamus as an excitatory signal.
The secondary (parvocellular) thalamus in turn projects into a cortex area that we want to call the secondary cortex.
Definition 2.11: Secondary cortex, secondary cortex clusters
The positive nuclear neurons of each primary cerebellum cluster project via the associated secondary (parvocellular) thalamus cluster into a cortex cluster of the secondary cortex.
The signal neurons of the secondary cortex clusters in turn project into a new cerebellum area via the bridge nuclei (bridge nucleus clusters) assigned to them. We also give this new area a name in order to shorten the text of future observations.
Definition 2.12: Secondary cerebellum, secondary cerebellum clusters
The signalling neurons of the secondary cortex clusters project via their associated bridging nucleus neurons into a cerebellum region, which we call the secondary cerebellum cluster.
It is well known (according to the author's theory) that all cortex areas produce two types of climbing fibres. The magnocellular climbing fibres have their origin in the activity neuron of the corresponding cortex cluster. The striosome system of the basal ganglia transforms this cluster excitation value into a magnocellular primary climbing fiber signal. This is converted into the required magnocellular secondary climbing fibre signals via central or sequential distribution. Therefore, each Purkinje group in the primary Cerebellum cluster has its own secondary (but still magnocellular) climbing fiber signal.
Every signal learned (imprinted) in the primary cerebellum cluster has an unmistakable digital signature. The dual digit at a k-th dual position has the value 1, if the corresponding moss fiber of the cortex signal belongs to the Purkinje group's own signal. If the signal of the corresponding moss fiber does not belong to the own signal but to the external signal, the value of this dual digit is zero.
We can now clearly describe the interconnected neurons of the primary cerebellum cluster, the secondary (parvocellular) thalamus cluster and the secondary cortex cluster. They all represent the same complex signal and have the same binary signature.
Theorem 2.23: Signature theorem
Each stored own signal of the primary cerebellum cluster corresponds to exactly one Purkinj group, one positive and one negative nuclear neuron and exactly one neuron in the secondary (parvocellular) thalamus cluster and in the secondary cortex cluster with the same digital signature. If a selection of signal neurons in the associated primary cortex cluster is sufficiently strongly active and if this neuron activity corresponds to a complex signal with the same digital signature, the Purkinje group recognizes this signal as its own signal (with a certain time delay) and causes the excitation of the associated thalamus neuron and the associated neuron in the secondary cortex. The prerequisite for this is that the current external signal does not overlap the intrinsic signal too much.
Each signal neuron in the secondary cortex thus corresponds to a complex signal with its own digital signature.
However, since each signal neuron of the secondary cortex also projects into the matrix system of the basal ganglia, exactly one parvocellular climbing fiber signal is generated for each of these signal neurons. This signal is always active (with a certain time delay) when the corresponding signal neuron in the secondary cortex is active. Therefore, we can assign exactly this complex signal to this climbing fiber signal. We can also assign exactly the same digital signature to this parvocellular climbing fiber signal.
Definition 2.13: Digital signature of the parvocellular climbing fibre signal
We assign to each parvocellular climbing fiber signal the same digital signature that the signal neuron in the secondary cortex has from which this climbing fiber signal was derived via the matrix system.
It is now becoming somewhat complicated, but a most important insight is imminent.
In the primary cerebellum there is a Purkinje group for each imprinted complex signal, whose digital signature corresponds to this complex signal. In case of recognition, the corresponding positive nuclear neuron excites exactly one signal neuron in the secondary cortex with the same signature. This sends out a climbing fibre via the matrix system, which also has the same signature. All these neurons are excited quasi synchronously (with a small time delay).
The climbing fiber and its generating neuron have an inherited commonality with all neurons of the signal chain. They received a common marker from their signal predecessors, i.e. their personal input suppliers. This marker is a mixture of various messenger substances that are distributed throughout the system. At least three of these messenger substances, which should be independent, enable them to find their way in space. This is due to the fact that each messenger substance has a place of origin around which it spreads e.g. by diffusion, whereby its concentration decreases. The concentration gradient is a function of the distance from the point of origin (or the area of origin).
If several such messenger substances with different places of origin overlap, each point in space has a typical mixture of messenger substances. This mixture marks the respective space point and thus distinguishes it from the other space points (hence the term "marker"). If this combination of messenger substances is passed on by the neuron to its connected partners, they can find their way back to the place of origin at the end of the chain. For this purpose, only the target-seeking axon must move in space in such a way that the marker concentration passed on to it ("inherited") as a search variable controls the movement of the axon in space in such a way that the extracellular marker combination corresponds as closely as possible to the internal one.
Examples of such procedures are described in the literature.
This means that the climbing fibre axon can find its partner of origin in the primary cerebellum. The author of this monograph now claims that every parvocellular climbing fiber signal does exactly this.
What reasons should there be for this?
First of all, it must be established that the Purkinje group, or more precisely its negative nuclear neuron, inhibits the associated magnocellular climbing fibre signal in the case of recognition.
The inhibition can be seen in the sketch 1.22 on page 40. And the more Purkinj groups of a cerebellum cluster have been imprinted with their own signals, the more often one of these signals is recognized after imprinting. This, however, statistically increases the number of Purkinj groups which inhibit all magnocellular climbing fibre signals. Therefore, an active climbing fiber signal becomes a rare event, because new, previously unknown signals of sufficient intensity and duration are becoming increasingly rare.
As a result, the synaptic contact between the Purkinje group and the associated magnocellular climbing fibre signal becomes increasingly ineffective.
This is the great hour of the parvocellular climbing fiber signal. Caused by the secondary cortex, it arrives at the nucleus olivaris and finds the neuron to which the original magnocellular climbing fibre signal had docked. This contact point has become free because the magnocellular climbing fibre signal was too often signalless and inhibited. This is where the new, parvocellular climbing fiber signal docks.
Thus the associated Purkinje group is now (additionally) supplied by its own parvocellular climbing fibre signal. Both signals - the output signal of the nuclear neuron and the parvocellular climbing fibre signal - have the same digital signature. Whenever the primary cortex sends a complex signal via the bridge nuclei into the primary cerebellum, which is the own signal of this Purkinje group, this Purkinje group is excited (with a short time delay) by the parvocellular climbing fiber signal of the corresponding secondary cortex. This is the phenomenon the author has long sought for in order to reproduce the Purkinj groups.
Each time this complex signal occurs in the primary cortex, the associated Purkinje group is replicated in the cerebellum via the parvocellular climbing fiber signal. If, during the activity of this climbing fibre signal, other signals are active which did not previously belong to the intrinsic signal of the Purkinje group, they are now additionally added to the previous intrinsic signal by long-term potentiation and long-term depression.
This makes the primary cerebellum more adaptive. The cause of the memory is no longer the single imprint in the imprinting period of about one second, but the continuous re-imprinting with essential signals.
However, it must be pointed out that the process of re-stamping described above is mathematically highly demanding. This is because during climbing fibre activity, new signals are added to the natural signal via LTP, which were active at the moment of imprinting. But also new signals are added to the external signal by LTD, because they were inactive at the moment of stamping. The result is a synaptic coupling strength that corresponds to a statistical frequency of this signal according to Hebb's principle. The own signal activity and the external signal activity of each bit of the digital signature depend on the statistical presence or absence of this signal.
Furthermore, the parvocellular climbing fiber signal does not have the same structure as the strio-somalic, magnocellular climbing fiber signal. The latter corresponds approximately to the neuronal system clock. Therefore, a mathematically and system theoretically exact replication theory has yet to be presented. Only the system-theoretical algorithm has been developed here. May others also help to cultivate this fertile field.
We summarize our findings in a new theorem. The most important finding is the gradual substitution of the magnocellular climbing fibre signals by the parvocellular ones.
Theorem 2.24. Substitution of the magnocellular climbing fibre signal by the parvocellular
When a Purkinj group in the primary cerebellum cluster recognizes its own signal, the strong inhibition of the associated permanently excited positive and negative nuclear neuron is eliminated. In the case of recognition, the negative nuclear neuron inhibits the associated climbing fibre signal in the olive, whereby the synaptic connection of the associated climbing fibre neuron to the Purkinje group becomes ineffective in the long term, since the strong climbing fibre excitation is always inhibited exactly when the Purkinje cells are inactive due to signal recognition. The output of the positive nuclear neuron of this Purkinje group reaches a signal neuron in a new secondary cortex area via the thalamus. Among other things, this signal neuron projects into the matrix system of the basal ganglia and generates a new parvocellular climbing fibre signal. This parvocellular climbing fiber signal replaces the now ineffective magnocellular climbing fiber signal and takes its previous place. Since this parvocellular climbing fibre signal is inevitably active exactly when the Purkinje group recognizes its own signal, a re-marking of the Purkinje group with the parvocellular climbing fibre signal is effected exactly in this recognition period. The substitution of the magnocellular climbing fibre signal by the parvocellular one (probably) takes place in the nucleus olivaris. The nuclear neurons are therefore co-excited by the parvocellular climbing fiber signal, the positive nuclear neuron reports this excitation to the thalamus.
Thus the learning process in Pontocerebellum is no longer limited to the first imprinting of the Purkinje group. Each time a signal is recognized, a re-marking is performed, so that the own signal components and the external signal components of the Purkinje group are updated over time by the re-marking.
Sketch 2.7 below shows the principle of climbing fibre substitution. It shows the state of the neuronal circuit after the complete substitution of all magnocellular climbing fibres of the striosome system by the parvocellular climbing fibres, which are formed by the signal neurons of the secondary cortex via the matrix system.
Sketch 2.7: Substitution of the magnocellular climbing fibers of the striosome system by the parvocellular climbing fibers of the matrix system
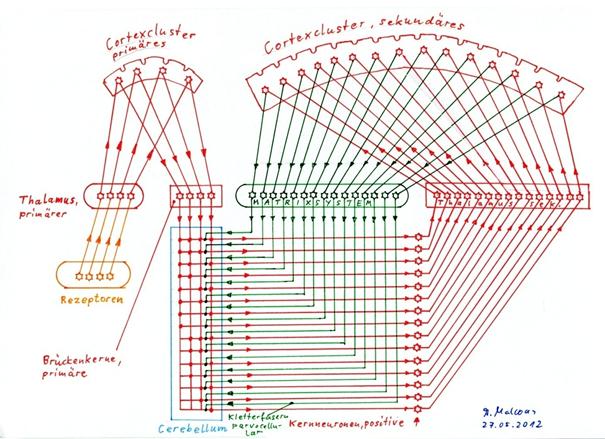
According to Theorem 2.6, the matrix system, which is shown in the above sketch as a green black box with exemplary 15 input and output lines, forms exactly one parvocellular climbing fiber signal for each signal neuron of a cortex cluster - here of the secondary cortex cluster - by quadruple negation of the cortex signal at an input signal. The relatively complicated circuit of the matrix system shown in sketch 2.2 is here simplified by a box in which exactly this signal conversion takes place. Only this way the climbing fiber substitution, which includes the transition from sketch 1.25 to sketch 2.7, can be presented in a comprehensible way.
The cerebellum is shown in simplified form in sketch 2.7. Only one Purkinje cell is assigned to each complex signal, basket, star and golgi cells are omitted. Similarly, only the positive, excitatory nuclear neurons are shown, otherwise the overview would be lost. The matrix system to the primary cortex is also missing. The complete striosome system is also omitted.
At this point we recall the change in the output of nuclear neurons during the imprinting process, which was described in Theorem 2.18. If this output is still the zero signal at the beginning of the imprinting process, at the end of the imprinting process it corresponds to the output produced by a final imprinted Purkinje group. The Purkinj groups subjected to re-stamping are already finish-stamped. For this reason, their output during the re-marking process does not differ (significantly) from the output in the period without a re-marking signal.
This is a very important finding. Since the parvocellular climbing fibre signal (with a short time delay) which reaches the (imprinted) Purkinj group through climbing fibre substitution is always active when the Purkinj group recognises its own signal, this climbing fibre signal must not interfere with the feedback of the recognition.
On the contrary: It is now completely irrelevant whether the Purkinje group recognizes its own signal or the re-imprinting climbing fibre signal is active. This is because an active re-imprinting climbing fibre signal on the already imprinted Purkinje group increases the activity of the star cells, basket cells and nuclear neurons which act as own signal detectors, but also the activity of the Purkinje cells which act as foreign signal detectors. However, since the basket cells and the star cells now inhibit the more strongly excited Purkinje cells more strongly, the resulting excitation of the Purkinje cells is almost unchanged.
We summarize our knowledge in our own theorem:
Theorem 2.25: Output of nuclear neurons of imprinted Purkinj groups after climbing fiber substitution with active climbing fiber signal
After climbing fiber substitution, an active parvocellular climbing fiber signal at a coined Purkinj group causes (roughly) the same output as the recognition of the intrinsic signal by this Purkinj group. The positive nuclear neuron reports the signal recognition to the thalamus with its excitation, while the negative nuclear neuron is also activated and inhibits the magnocellular climbing fiber signal. The output during re-imprinting is (almost) identical to the output during signal recognition.
About the same output may be interpreted to mean that a small change in the rate of fire is possible, but does not change the detection.
However, the algorithm of repopulation is not only associated with a real circuit change in the cerebellum, but also with a paradigm shift.
As an example we consider a Purkinje group before the substitution of the magnocellular climbing fiber signal by the parvocellular one. When this Purkinje group recognized its own signal, it immediately inhibited the magnocellular climbing fiber signal from the striosome system in the olive. Depending on whether the magnocellular climbing fiber signal was obtained by central distribution or by sequential distribution, the corresponding distribution neuron was inhibited in the case of recognition. This inhibition of the magnocellular climbing fiber signal remains even after substitution. By maintaining the inhibition of the striosomal climbing fiber signal, the same signal is prevented from imprinting a new, free Purkinj group as before. Multiple imprints are thus prevented in the same way as before. The Purkinje group thus continues to inhibit the striosomal climbing fibre signal in the case of detection, even if it is no longer reached by this striosomal climbing fibre after climbing fibre substitution.
The question of whether the new, parvocellular climbing fibre signal of the matrix system, which now-more docks in the nucleus olivaris to the responsible (previous) climbing fibre neuron, is inhibited there by its own Purkinj group (or its negative nuclear neuron) in the case of recognition in the same way as the magnocellular one is inhibited, will be answered later. Such an inhibition seems absurd, because both are synchronously active (except for a small time delay). However, the phase shift of the signals cannot simply be neglected due to the longer propagation paths. Therefore we postpone the answer until later, when we have to discuss the functionality of a circuit that is a kind of inverse video memory. With such a circuit the cerebellum could play back previously stored video sequences in the same way, e.g. during a dream phase.
We summarize the previous findings in a separate theorem.
Theorem 2.26: Persistence of the inhibition of the striosomal climbing fiber signal after climbing fiber substitution
After substitution of the magnocellular, striosomal climbing fiber signal of a Purkinj group in the nucleus olivaris by the parvocellular climbing fiber signal of the matrix system, the existing inhibition of the striosomal climbing fiber signal by the Purkinj group remains in the case of recognition. Thus, multiple imprinting of the intrinsic signal of this Purkinje group in the cerebellum cluster by the magnocellular striosomal climbing fiber signal is prevented.
In principle, only little changes for the rest of the cerebellum after the climbing fibre substitution. The striosomal, magnocellular climbing fibre signal remains responsible for the imprinting of new, previously unknown signals, if they are imprintable in intensity and duration.
Only those Purkinj groups in which the climbing fibre substitution from magno-cellular to parvocellular has already taken place are capable of replication. Therefore, the striomome system continues to be responsible for re-learning signals in the cerebellum, while the matrix system is responsible for refining and updating what has been learned.
However, the process of re-stamping must be analysed more closely from a systems theory perspective.
During stamping, the synaptic coupling of the intrinsic signal detectors changed from the starting value ½ to 1 within one second due to long-term exponentiation. The synaptic coupling of the extraneous signal detectors changed from 1 to ½ due to long-term depression in the stamping second.
With the re-stamping process everything goes much slower and in smaller change steps. The author suspects that the coupling value can only change in the percentage range during restrike minting. If a parallel fiber is active in the cerebellum that does not belong to the embossing signal, the active parvocellular climbing fiber signal increases the coupling for the own signal detector (e.g. basket cell) from the value ½ to the value 1 as before. The previous long-term exponentiation therefore remains undiminished.
If, however, this parallel fiber is part of the intrinsic signal and was inactive, i.e. signalless, during re-stamping, the coupling value must decrease again. However, this reduction does not occur abruptly from 1 to ½, but much more slowly. First learning is therefore fast, relearning slower. An example may illustrate this. If a cat or a dog begs for food while its owner is eating at the table, and the animal begs for a bite of food for the first time, it will beg for food again and again while eating at the table. It takes much longer to break the habit of begging. The first learning process "begging is rewarded with food" is very difficult to transform into the learning process "begging is never rewarded with food".
We put this knowledge into our own theorem.
Theorem 2.27: Stepwise change of the synaptic coupling during replication
The strong increase in synaptic coupling in the cerebellum's own signal detectors caused by long-term potentiation can only be reduced step by step by means of repopulation. Similarly, the strong decrease in synaptic coupling in the foreign signal detectors of the cerebellum caused by long-term depression can only be increased again step by step.
We want to understand the reversal of LTP or LTD as inverse LTP or inverse LTD. However, other macromolecular processes are used here, which neurologists and other researchers must first determine. The author only suspects that the inverse processes are much slower. However, this is an unproven hypothesis. It should be remembered that the author is a mathematician, so he cannot provide such proof himself.
ISBN
978-3-00-037458-6
ISBN 978-3-00-042153-2
Monografie von Dr. rer. nat. Andreas Heinrich Malczan