Theory of the neuronal circuity of the brain and analytical thinking
ISBN
978-3-00-037458-6
ISBN 978-3-00-042153-2
Monograph of Dr. rer. nat. Andreas Heinrich Malczan
Part 2.6 The symbiosis of cerebellum and hippocampus
After the theory of imprinting Purkinje cells of the cerebellum has been explained, a still completely open problem of the permanent storage of complex signals in the cerebellum must be pointed out. According to generally accepted theory, long-term depression or long-term potentiation in nerve cells only occurs when a tetanic stimulus (the climbing fibre signal) acts together with the input signal to be learned for at least the duration of about one second. Both the climbing fibre signal and the signal to be imprinted must act simultaneously and together for at least one second.
So let's go to the movies. Moving images - each image affects our retina by about 1/30 second. Fast moving scenes are still perceived by us. And even after a long time, we recognize partial scenes when we see them again. Neurologists have observed an increase in temporal resolution in young people, especially with extremely fast video clips or computer games, where older people are visually overwhelmed.
But how can our cerebellar memory learn and later recognize images that only affected us for a small fraction of a second? LTP and LTD are not possible here, for this purpose the signal exposure time would have to be much longer!
So where in our brain is it ensured that, for example, an image with an exposure time of a thirtieth of a second can still be stored?
This question has remained unanswered in international literature to date and (in the author's opinion) is scientifically answered for the first time in this monograph. Perhaps this question has not yet been asked?
How do you actually extend the duration of a signal? Nature shows us how. For example in German Bavaria or in Polish Zakopane. High mountains reflect the sound and create an echo. If the distance to the reflecting mountain wall is favourable, the echo begins exactly when the original signal stops. With a bit of luck you can hear several echoes. With even more luck, an echo starts exactly when the previous echo just stops. This way you can hear an uninterrupted sequence of echoes without much distance between the partial echoes.
We want to call a circuit that repeats a signal as an echo (even several times) an echo generator. So where in the brain is there an echo generator? According to the author, the neural echo generator is located in the hippocampus. But before explaining the theory of the hippocampus as an echo generator, it must be pointed out that this is not the only task of the hippocampus. The function of the hippocampus as an echo generator is only one of several tasks of the hippocampus.
In every good neurology textbook you will find a graphic representation of the hippocampal structure and the circuitry. From a systems-theoretical point of view, the most meaningful one can be found in the "Taschenatlas Anatomie in 3 Volumes" by Werner Kahle and Michael Frotscher of the Thieme-Verlag on page 237. But this time, this drawing should not be reproduced as a picture quotation. Instead, we want to derive the circuit of the hippo campus theoretically and then compare it with this illustration.
First, an echo generator needs a delay line.
A delay line is comparable to a narrow road with speed limits. All cars suddenly have to brake hard. Their distance to each other is noticeably reduced. Due to the low speed, the vehicles pile up. Once the bottleneck has been passed, each car has to show a time delay that it would not have suffered without this bottleneck. The car driver then speaks of a "delay".
On a delay line the action potentials spread out with clearly reduced speed. Depending on the length of the delay line, they arrive at the end with a significant time delay. If a signal has passed a delay line, it arrives at the end of the delay line with a delay, quasi as an echo of the original input signal.
The author of this monograph thus interprets the hippocampus as an echo generator that generates echoes to each input, which therefore have a time delay to the original signals.
A neuron feeds its output into this delay line. The delay line is an axon whose neuron is a granule cell of the hippocampus. The granule cells are present in the dentate gyrus of the hippocampus in millions and receive their input from the association areas via the enterohirnal cortex via subiculum. In the dentate gyrus, each input line annexes a granule cell whose very long and unmyelinated axon forms the so-called moss fiber. Exactly this moss fiber is our delay line. First, it has a considerable length, because it projects to the CA3 region of the hippocampus. Secondly, it is unmyelinated. When the input reaches the granule cell and triggers an action potential in it, this action potential will spread out on the moss fiber at relatively low speed.
Let l be the length of the moss fibre and v the velocity of the action potential on it. Let l = 100 millimetres and v = 0.1 m/s as an example. Per second an action potential on the moss fibre travels 0.1 metre, i.e. 100 millimetres. (Very probably the speed is even lower:)
We divide the moss fiber hypothetically into 10 parts of equal length and tap the moss fiber with one neuron in each of the resulting 10 division points.
Therefore we now have 10 output neurons N1 to N10. They all tap into the moss fiber as input. The distance from one output neuron to an adjacent one is 10 millimeters. For this distance, an action potential needs the time of 0.1 second.
The input neuron of the granule cell supplies this moss fiber with a signal that lasts 1/10 second and has a fire rate of 100 action potentials per second.
This signal propagates unchanged on the moss fiber because the granule cell outputs each incoming action potential directly as output action potential on its moss fiber axon. Then the following will happen:
· After 1/10 second waiting time, the output neuron N1 fires a sequence of 10 action potentials at intervals of 1/100 second, which lasts exactly 0.1 seconds. This time delay is caused by the fact that each action potential has to travel the distance of 10 millimeters from the granule cell to the neuron N1, taking exactly 0.1 seconds.
· After a 2/10 second waiting period, the output neuron N2 fires a sequence of 10 action potentials at intervals of 1/100 second, which lasts exactly 0.1 seconds. This time delay is caused by the fact that each action potential has to travel the distance of 20 millimeters from the granule cell to the neuron N2, which takes exactly 0.2 seconds.
· After a 3/10 second waiting period, the output neuron N3 fires a sequence of 10 action potentials at intervals of 1/100 second, which lasts exactly 0.1 seconds. This time delay is due to the fact that each action potential has to travel the distance of 30 millimeters from the granule cell to the neuron N3, taking exactly 0.3 seconds.
· and so on
· After a 10/10 second waiting period, the output neuron N10 fires a sequence of 10 action potentials at 1/100 second intervals, lasting exactly 0.1 seconds. This time delay is due to the fact that each action potential has to travel the distance of 100 millimeters from the granule cell to the neuron N10, taking exactly 1.0 seconds.
So, to sum up: The ten output neurons that tap into our moss fiber create echoes of our input signal. But there is a time difference of 0.1 seconds between the first, the second and each subsequent echo. This (randomly) corresponds to the duration of our input signal.
So we have generated 10 echoes so far, each echo immediately follows its predecessor echo. Now we have to explain where our 10 output neurons are located in the hippocampus. We decide: The output neurons for the different individual echoes should be the pyramid cells of the CA3 region of the hippocampus. However, we require that these output neurons all have to tap the same moss fibre and that they are all only allowed to tap the same moss fibre.
The echo generation is shown schematically in the following sketch using an example with 5 neurons that generate 5 echoes. The input comes from the left and consists exemplarily of exactly 5 action potentials. These move on the axon acting as delay line, which is shown in red and corresponds to the moss fiber. At each branching point a neuron is shown in green, which taps the moss fiber. The moss fiber signal propagates at each branching point both on the moss fiber and on the branch shown in green. But the speed of propagation of the action potentials on the axons shown in green is much higher. Therefore, the output of the system now hits as a consequence of primary echoes. The first partial echo provides the first neuron (green) on the far left of the image. The movement of the action potentials is shown in time, we see the system of delay line and the faster collector lines at ten different times, in which the action potentials spread from left to right.
From about Fig. 5 on, the first echo signals (on the green axons) have arrived on the left. In all following different points of time, a signal is constantly arriving on the green output lines on the left. This only ends when there are no action potentials on the delay line shown in red.
A summary of the many primary individual echoes into a total echo is not shown in the following sketch, but takes place in the hippocampus in the CA1 region.
Sketch 2.5: Echo generation on a delay line
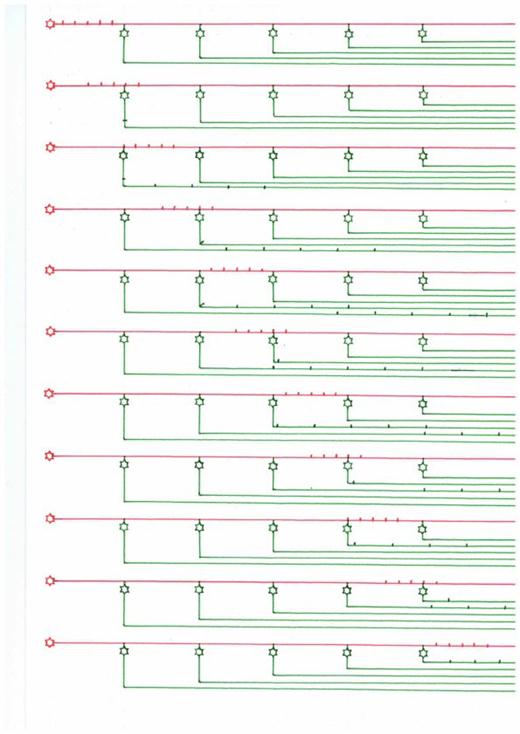
Let us again refer to the example chosen at the beginning with the ten individual echoes that are generated on a moss fiber of the hippocampus.
However, since there are millions of moss fibres, each moss fibre has its own separate and very private echo system from the CA3 pyramid cells that it taps.
So we have 10 consecutive single echoes of our input signal. As can be seen in the neuronal circuitry of the hippocampus - available in every better textbook - the axons of the CA3 pyramid cells move to the septum on the one hand and deliver the corresponding echo sequence there via the (in our case ten) output axons.
At the same time, however, the output of each CA3 echo cell is routed via an axon collateral to the CA1 field of the hippocampus. However, these axons are myelinated. Therefore there is no (measurable) time delay. And on each pyramidal cell of the CA1 region, many axons of the CA3 region converge. Therefore we demand: All ten output axons may be split into two axon branches each. One axon branch projects to the septum, the other to exactly the same pyramid cell of the CA1 region. We want to call this pyramid cell an echo-summing neuron. It combines the ten echoes arriving at it with a time delay into a total echo. This works because the feeding echoes are myelinated and arrive at the echo-summing neuron almost without time delay.
The result is the desired: An input of 0.1 seconds duration with a fire rate of 100 Hz results in a continuous signal of 1 second duration and a fire rate of 100 Hz in the corresponding pyramid cell of the CA1 region of the hippocampus. The output of the CA1 neuron thus lasts ten times as long as the original input from the association fields of the cortex. We consider that the tenfold signal extension is due to the fact that we used a moss fibre of 100 millimetres as an example. This extended the original signal to a total of one second. In the same way, we could have used a moss fibre twice as long or half as long. For example, if the speed of propagation of the action potentials we hypothetically assumed was only 10 millimeters per second instead of 100 millimeters per second, the signal composed of 10 part echoes would have a time length of 10 seconds.
It is the principle: From a short input signal (with a minimum-length of here exemplary 0.1 seconds) the hippocampus generates on the one hand a group of echo sequences in the septum, on the other hand a group of continuous signals of strongly extended duration. The axon branches that project from the pyramid cells of the CA1 region to the pyramid cells of the CA3 region, so that the many, temporally offset individual echoes can be combined into a temporally strongly stretched signal, are called Schaffer collaterals in the classical neurological literature. In contrast to the moss fibres, they should be myelinated. However, any mathematician or physicist can develop a model that uses only weakly myelinated Schaffer collaterals and produces approximately the same result. In this case, the output result would be a sequence of consecutive echoes with certain additional time intervals, similar to the neural system clock.
The hippocampal beat, the hippocampal theta, cannot yet be discussed here. Perhaps its explanation will be possible later, when the necessary system-theoretical foundations have been laid. However, it should be pointed out that the hippocampal theta is a consequence of the loose synchronization of the neuronal system clock in the substantia nigra pars compacta. This could be reinforced by the existence of a recurrent inhibitory connection in the opposite direction to the normal signal direction in the matrix system of the basal ganglia, since this would also cause a forced oscillation in the matrix system through feedback. This, however, requires the basic neural interconnection of the basal ganglia system with the limbic system, which cannot be presented here yet.
Sketch 2.6: The principle circuit diagram of the hippocampus according to A. Malczan - status: January 2012
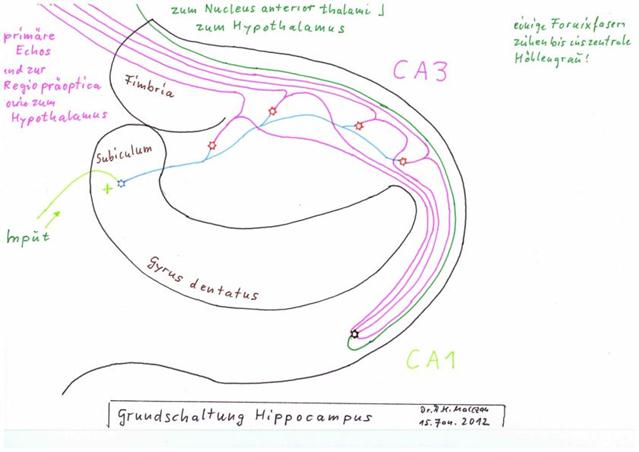
Legend:
- Input: yellow-green, via subiculum
- Moss fibre (delay line): blue
- Output lines for primary time-delayed echoes: violet across the fimbria, moving mainly to the preoptic region and the hypothalamus
- Schaffer collaterals, also violet, conduct the primary and time-delayed echoes to the echo integration neuron in the CA1 region (green), where a secondary echo of much longer duration is generated from these many primary echoes
- Output line of the secondary echo green, mainly to the anterior thalamic nucleus and the hypothalamus
- some output fibres stretch into the central cave grey
(Source for the texts: "Taschenatlas Anatomie in 3 Bänden" by Werner Kahle and Michael Frotscher of Thieme-Verlag)
As can be seen in the figure on page 237 of the "Taschenatlas Anatomie" by Kahle/Frotscher, an input signal - i.e. of each granule cell - is not only accompanied by an output signal from the CA1 region, but a whole chain of neurons generates a sequence of strongly extended signals of different duration for the same and identical input. The duration of the "time-stretched" output signal varies depending on how many adjacent CA3 neurons project together onto an output neuron of the CA1 region.
Since the principle of echo formation by means of delay lines, which are realized with the help of un-myelinated axons, is found in the brain in several subsystems, we want to introduce two new terms to simplify the future expression.
Definition 2.3: Primary and secondary echoes of a signal
If a signal is repeated by an echo that represents only the time-delayed original signal of equal length in time, we call this echo the primary echo.
If several such primary echoes are superimposed to form a new signal with as little time interval as possible, we call the signal resulting from the superimposition a secondary echo.
A secondary echo has a much longer signal duration than the original signal.
We summarize our findings in a new theorem.
Theorem 2.12: Echo generation and temporal signal stretching in the hippocampus
An input signal to a neuron of the subicle of the hippocampus suffers a propagation delay during its propagation on the (almost) unmyelinated moss fibre of this neuron, which is (approximately) proportional to the distance already travelled on this moss fibre. By tapping this moss fiber, the pyramidal cells of the CA3 region of the hippocampus generate a series of primary echoes of the input signal, which are time delayed with respect to each other.
The sequence of primary and time-delayed echoes of the different CA3 pyramid cells of the same moss fibre reaches the septum on the one hand.
On the other hand, in the CA1 region of the hippocampus, the different primary and time-delayed echo signals of the CA3 pyramid cells of the same moss fiber converge on a population of CA1 pyramid cells, so that their output produces the time-stretched input signal (secondary echoes). The total duration of the secondary echo of a given CA1 pyramid cell depends on the mean velocity of propagation of the action potentials on the unmyelinated moss fiber and on the distance between the first and the last CA3 neuron whose output converges on a given CA1 neuron.
As a consequence of primary echoes, the output of the CA3 region mainly reaches the septum and moves to the preoptic region and the hypothalamus, among others. This output is mainly required for short signals to determine short-term temporal changes. For example, movements in the visual range can be detected by comparing the original signal and the primary echoes. But perhaps more about this later.
The output of the CA1 region reaches mainly the nucleus anterior thalami and the corpus mammilare as well as the hypothalamus as a result of secondary echoes. There, therefore, the strongly time-stretched signals (secondary echoes) generated by a very short signal are available, which were generated by simply stringing together the likewise short (primary) echoes from the CA3 region. With these signals, which now last significantly longer than one second, the cerebellum can achieve the imprinting that would not have been possible without time-expanded signals. The cerebellum is reached by these signals because the output of the nucleus anterior thalami reaches the cerebral convolutions of the cinguli gyrus, which in turn (like all cerebral cortices) project to the cerebellum via the bridge nuclei.
It can therefore be assumed that the cerebellum and the hippocampus form a symbiosis. Both are interdependent, both are made for each other. From an evolutionary point of view, both must have been created at about the same time. One should not forget the objection raised at the beginning that echogenesis and temporal signal stretching are only a partial task of the hippocampus. The author of this monograph has already set his sights on a second task and will present it in the third chapter of this monograph.
Here we remember the question, which has so far only been answered unsatisfactorily, why a climbing fibre contacts several (depending on the literature reference one, three, eight or even 12) Purkinje cells and why exactly the Purkinje cells contacted by it contact the same positive and also the same negative nuclear neuron in the cerebellum nucleus. The question why such a group of Purkinje cells ends up with a Golgi cell was answered: This causes the transmission inhibition of the moss fiber signals in case of recognition.
But what sense should it make to imprint three, eight or even 13 neighbouring Purkinje cells with the same own signal? Is not one "spare cell" sufficient as a "failure reserve"?
Here we remember the echo theory of the hippocampus. It is known that the hippocampus contains the granule cell bodies of the granule cells of the dentate gyrus in the stratum granulare. Here we read literally in the textbook "Anatomy Volume 4" by Graumann/Sasse on page 328:
(quotation begins:)
"Stratum granulare:
It contains very densely packed pericardium of the granule cells of the dentate gyrus. In size and shape, these resemble the granule cells of the cerebellar cortex - and not the larger and variably shaped neurons of the granule cell layers of the isocortex.
(end of quote)
This addresses the anatomical similarity of the granule cells of the hippocampus and the cerebral bellum. Therefore, similarity can be expected from the associated axons. This seems to be the case. Both types of axons are unmyelinated and very thin. It can therefore be assumed that the action potentials spread relatively slowly both on the moss fibers of the hippocampus and on the parallel fibers of the cerebellum. It should again be clearly remembered that Valentin Braitenberg already suspected in the last century that a relatively low speed of propagation of the action potentials on the parallel fibres was used in the cerebellum for time measurement purposes. The author of this monograph is a great admirer of Valentino Braitenberg and takes the liberty of updating and supplementing this theory of Valentin Braitenberg, which has perhaps been somewhat forgotten.
So we hypothetically assume that a climbing fiber signal would imprint a Purkinj group with the same inherent signal.
For embossing, both the climbing fibre signal as a tetanic stimulus and the signal to be embossed must act jointly on the parallel fibres for a period of at least one second. For example, a living being could explore its immediate environment relatively slowly and without stress and "learn" the obstacles present in this environment. We take a concrete object of the environment as an example signal. There would therefore be plenty of time for its imprinting.
Later, at a time t, this signal may be applied as an input to the moss fibres and the associated granule cells of the cerebellum for the very short time period of, for example, 0.1 seconds.
For example, now, after the relatively slow exploration of the surroundings, a sudden danger could make a quick escape necessary. If our example object is suddenly in the way, it can only be "circled" if it is detected. For the recognition only the time of 0.1 seconds is now available, because the escape must be fast. Such a fast recognition is in itself no problem. The problem lies rather in remembering the selected escape route. This requires a special solution.
The rate of fire of this very short signal of recognition is exemplary 100 Hz. Therefore the signal consists of exactly 10 action potentials, which follow each other in a time interval of 1/100 second. Each action potential rises at the granule cell laxon of the Purkinje group which is characterized by this signal. The granule cell laxon is divided into a left and a right parallel fiber branch. The action potential runs along each of these branches. Thus, a short signal consisting of 10 action potentials runs along each half branch of the parallel fiber. It meets the first Purkinje cell. Since the signal consisting of many activated parallel fibres is the Purkinje cell's own signal, as agreed, it interrupts the inhibition of the associated output neuron of the cerebellar nucleus for a signal duration of 0.1 seconds. But the signal continues to spread out on the parallel fibres and hits the second (left or right) Purkinje cell. Their inhibition now also fails. However, we remember that a common group of Purkinje cells contacts a common glutamatergic output neuron and also a common GABAergic projection neuron of the cerebellar nucleus. This was already shown in the dissertation of Susanne Kamphausen: "The ratio of Purkinje cells to cerebellar nucleus neurons was estimated to be 10:1". A parallel fibre thus contacts several Purkinje cells, but also several Purkinje cells contact the corresponding output neurons of the cerebellar nuclei. We assume that exactly those Purkinje cells contact common output neurons that are activated by the same climbing fiber.
Consequently, the next Purkinje cell also stops inhibiting the same output neuron of the cerebellar nucleus, exactly for the duration of this signal (here 0.1 seconds). All this is repeated until the last Purkinje cell of the same Purkinje group is reached.
However, this does not yet bring any profit, because of the related Purkinje cells in our example - each one separately - the inhibition of the cerebellar nucleus output neuron would only stop for 0.1 seconds and then resume inhibition. This is why the basket cells now begin their previously unexplained activity.
Again, we would like to quote from the textbook "Anatomie Band 4" by Graumann/Sasse. On page 279 it says:
(quotation begins:)
"Basket cells are medium-sized inhibitory interneurons They are located in the stratum moleculare, near the perycaryotes of the PURKINJE cells. Their dendritic tree extends into the stratum moleculare, their axon spins around perycaryons and axon mounds of eight to ten neighbouring PURKINJE cells with numerous fine branches. Basket cells are responsible for the lateral inhibition of PURKINJE cells."
(end of quote)
A basket cell thus contacts eight to ten Purkinje cells. This had been somewhat neglected in the beginning. Each Purkinje cell has its own basket cell whose axon contacts each Purkinje cell of the same Purkinje group.
The author of this monograph assumes that each basket cell jointly inhibits all Purkinje cells belonging to the same group as long as the imprinting signal is applied to this basket cell. Thus, as long as the imprinting signal travels along the parallel fibers and excites the dendrites of the different basket cells, they will inhibit all Purkinje cells of that group. A fast inhibition is necessary, the inhibition signal must be significantly faster than the action potentials on the slower parallel fibres. A certain myelinisation would therefore be advantageous, but thicker axons might also contribute to this.
Overall, there is a significant extension of the inhibition loss of the Purkinje cells in question, because all Purkinje cells of the same Purkinje group project both on the same glutamatergic and the same GABAergic output neuron of the cerebellar nucleus. Thus, the cerebellum output is significantly increased - quasi by echo formation along the parallel fibres. It is the same principle of action as in the hippocampus. And it means that the cerebellum can now use its own output as input because every signal, no matter how short, is stretched to a formable length of about one second. Therefore, if many Purkinje cells belong to a Purkinje group, the more temporally stretched output of the cerebellum can be projected via the thalamus to the cortex and from there via the bridge nuclei back into the cerebellum to be combined into new complex signals. The imprinting ability of the cerebellum output was a basic prerequisite for the cerebellum to use its own output as input. Only then did the principle of associative matrices in the cerebellum become associative for short signals that are not actually capable of being imprinted. However, the precondition was that the cerebellum output could reach the cerebellum as input again.
The example of our fleeing creature makes it clear: If the escape was successful, it automatically leads to learning the escape route. Even though the individual signals could only have an effect for 0.1 seconds each during the escape, they were temporally stretched by the echo formation within the Purkinje group and were thus signals that could be imprinted. They did not only reach the thalamus and the cerebral cortex, but also returned to the cerebellum via the bridge nuclei. In this way the (possibly randomly) chosen escape route was learned.
The resulting evolutionary advantage was undoubtedly there. Short non imprintable signals were prolonged to an imprintable period of time and used again for imprinting in the cerebellum. The recognition of very short signals in the cerebellum could trigger associative reactions because the signal output from the cerebellum was stretched in time
The Purkinj groups were not always present and developed gradually during evolution. This is why some authors state that only three Purkinje cells from the same climbing fibre are provided, while others speak of 8 or even 13. Here it can be assumed that the number of Purkinje cells of a Purkinje group increased gradually during evolution.
Theorem 2.13: Time expansion of a short signal by a Purkinje group
A Purkinje group of Purkinje cells extends a very short signal, which is the group's own signal, by echoing along the parallel fiber axons, with each Purkinje cell and the basket cell belonging to it generating a partial echo. The cause of the echo formation is a reduced speed of propagation of the action potentials along the parallel fibres, whereby they reach the different Purkinje, Basket and Star cells of the same group at different times.
Because each basket cell belonging to the group inhibits all Purkinje cells belonging to the group extremely quickly in the case of recognition, their excitation does not occur when the basket cells have recognised their own signal and this is at least as strong as the possible foreign signal.
For the total duration of the echo signals (and thus the secondary echo), this basket cell population of the group inhibits all active Purkinje cells of the group. The output of all Purkinje cells of the group converges to an excitatory output neuron of the cerebellar nucleus, which, among other things, moves to the thalamus. On the other hand, this output converges to a common inhibitory output neuron, whose integrated output suppresses the climbing fiber signal in the olive and inhibits it in the nucleus ruber. The cere-bellum output on a very short input signal is transformed by the Purkinje group into a time-stretched output. Thus, the transformed output is imprintable, while the output without transformation would be too short to cause imprinting in the cerebellum as input. The more Purkinje cells belong to a Purkinje group and the slower the action potentials propagate along the parallel fibres, the longer the temporal stretching of the original input in the case of recognition.
We recall the hypothesis that the cerebellum developed successively. In the beginning there was only one Purkinje cell, later a second one was added. At first, it was a kind of "failure reserve" that continued its task after the neuronal death of the first Purkinje cell. Later, other Purkinje cells were added, which all learned the same signal.
With the development of golgi cells, the principle of inhibition of transmission was developed, whereby initially only the granule cells were probably inhibited.
At this stage of development, the golgi cells divided the cerebellum cluster into Purkinj groups, each of which could now learn a different signal. At this time, the signals spread only along the parallel fibres. A moss fibre system simply did not exist at that time.
And there the relatively low propagation speed - which was indispensable for echo formation and temporal stretching - proved to be a system-theoretical obstacle.
One consequence of the assumed time delay of the signal propagation along the parallel fibers was that time was needed to remember. The parallel fiber signal had to travel along the parallel fibers until it (finally!) encountered a Purkinj group whose own signal (at least half) matched the parallel fiber input. The more different signals had to be checked for agreement beforehand, the more time passed until they were recognized. And again, evolution was struggling with itself. A very long length of the parallel fibres allowed the learning of many different intrinsic signals. At the same time, the relatively low propagation speed and the increasing length of the parallel fibres greatly increased the average recognition time for signals.
If, for example, 10 different signals were stored during active learning, the first signal learned could be detected the fastest. Every signal learned later followed later in the Purkinje group order. More time passed until the parallel fiber signal arrived there and the Purkinje group actively responded. Signals were thus stored sequentially in the Cerebellum if their input source was in the same cortex cluster.
Now it also becomes clear that the number of Purkinje cells that belong to a Purkinje group is a variable parameter of the cerebellum. It is known that such a group is flanked by two externally arranged golgi cells. The more Purkinje cells belong to this group, the greater is the temporal stretching of the possibly relatively short-term parallel fiber signal due to the echo formation of the Purkinje cells of this group. However, in the primitive Cerebellum (without moss fibres) this is bought by the fact that the search for an own signal in the Purkinje cell set of the Cerebellum cluster was significantly slower. The fewer echoes are generated to a parallel fiber input, the faster the Cerebellum cluster delivered the matching Purkinje cell for a certain signal, whose own signal corresponded best to this input. Thus, the speed of memory and the ability to combine extremely short signals were controversial, an improvement in ability in one direction worsened ability in the other direction.
In order to compensate for the slowness creeping into the system due to the lower propagation speed along the parallel fibres, nature thought up a faster supply of the Purkinj groups with the necessary signals. It invented the faster moss fibres, which brought all signals as quickly as possible to all Purkinj groups at high speed. Therefore, each Purkinj group needed its own parallel fibres. These were formed by the granule cells which were located within the spatial extension of the Purkinj group. The axons rising from them split left and right into the parallel fibres to supply all group members (Purkinje cells, basket cells, star cells, but also the Golgi cell at the end of the group) with the input, which arrived at high speed from the moss fibres. We have called this population of parallel fibers direct parallel fibers.
The signals of the indirect parallel fibers deliver the same signals with a certain delay, which in turn means that short input signals have also been stretched to a much longer time length. Therefore, the ability to learn and recognize short signals depends on the average total length of the parallel fibers. The longer this length, the more an incoming input from the indirect parallel fibres is stretched in time, because the associated indirect granule cells received their input signal via the moss fibre before the incoming climbing fibre signal from the striosome system could suddenly stop the signal propagation on the moss fibres to the more distant neighbouring groups by strong excitation of the golgi cells.
This in turn meant that the recognition of a signal not only required the inhibition of granule cells by the Golgi cell of the Purkinj group in question, but also the suppression of further signal propagation along the moss fibres. Therefore, it proved useful to include the moss fibres in the Golgi inhibition so that the neighbouring groups did not receive signal input via the parallel fibre lines formed by the neighbouring Purkinj groups. In this respect, the double transmission inhibition is favourable: Ascending granule cell laxons and moss fibres of the group are both inhibited.
We put our knowledge into a new theorem.
Theorem 2.14: The need for own direct parallel fibers in each Purkinj group
Due to the high propagation speed of the signals along the moss fibres and the slower propagation speed along the parallel fibres, each Purkinje group needs its own direct parallel fibres, whose generating granule cells are spatially located within this Purkinje group. Only this ensures that all Purkinje groups of the entire Cerebellum cluster can start analyzing a moss fiber signal at about the same time. This is because the signals of the indirect parallel fibers of the neighboring, sometimes even more distant Purkinj groups arrive with a time delay, so that it became advantageous to obtain the same signals via the direct parallel fibers without time delay. This was the only way to avoid a considerable time delay in signal analysis.
Thus, the longer the parallel fibres became during evolutionary development, the more necessary it became to ensure the signal supply via a sequential distribution system, which was extremely fast. This fast distribution system for the cerebellum input was the moss fiber system. It eliminated the disadvantage of the time delay of the signals along the parallel fibers, because now each Purkinj group had its own input lines to the moss fiber system.
But why did the parallel fibres not become shorter again after the invention of the moss fibre system, if the long length was the reason for the greater time delay? This shows that advantage and disadvantage are often closely related.
It is precisely this time delay that is advantageous for extremely short signals, whose duration of influence on the cerebellum neurons is prolonged by the superposition of the signals of the direct and indirect parallel fibers.
Theorem 2.15: Time expansion theorem of the cerebellum
If the signal propagation velocity along the moss fibres is k times greater than the propagation velocity along the parallel fibres and a climbing fibre signal from the activity neuron via the striosome system, the nucleus ruber and the olive to the Purkinje cells requires a time span of about Δt, the length of an input signal in the cerebellum is temporally stretched by a maximum of k*Δt after a necessary previous signal pause. This is due to the fact that after a signal pause the climbing fiber axon also becomes signalless. A cortex signal arriving after this signal pause is transformed by the activity neuron of the cortex cluster via the striosome system into a climbing fiber signal, which becomes effective in the Purkinje cells with the time delay Δt. The same cortex signal reaches the moss fibres of the cerebellum via the bridge nuclei almost without time delay and is passed on to the granule cells, which transmit it to the parallel fibres. However, since the signal travels along the parallel fibres k times slower, it travels along the parallel fibres it reaches for the time period k * Δt and is thus extended in its temporal length by the value k*Δt. Therefore, even extremely short signals can be detected by the cerebellum and the output can be transformed into an imprintable temporal length. Furthermore, this means that the double forwarding inhibition cannot prevent the signals arriving after a signal pause from running along the parallel fibers for the time period k*Δt and leading to imprinting or signal recognition. This is one (of several) basis for the temporal short-term memory of the cerebellum.
It should be clearly pointed out that a useful temporal stretching of input signals by superimposing direct and many indirect parallel fibres is only possible if a signal pause, i.e. a signal-free time period, was previously present.
First of all, it must be ensured that there is no longer a climbing fibre signal that would cause the Golgi cells to inhibit signal transmission. Then a signal flows from the cortex to the cerebellum, whereby the signal reaches the parallel fibres via the bridge cores and spreads on them before the magnocellular climbing fibre signal formed from these cortex signals even reaches the Purkinje cells.
We want to call signals that are preceded by a sufficiently long signal pause start signals. For example, the first letters of words are such starting signals, because we learn words as units separated by pauses. Only later do we learn the ability to make these pauses between words shorter or even to omit them. If we use the temporal order as a basis, we can describe different, chronologically successive signals with topological order terms, which are defined below.
Definition 2.4: Start signal and following signal, preceding signal, following signal
Let S be a complex signal preceded by the zero signal. Then we call S a start signal.
He said S1 was a signal. If a signal S2 starts exactly when the signal S1 ends, we call S1 the predecessor signal of S2 and S2 the successor signal of S1.
The cerebellum can now store a new type of signal by applying time dilation via the parallel fibres. For the first time it is possible to combine a start signal and its successor signal to a new complex signal.
Because of the immense importance of this ability, we define the concept of the start signal sequence.
Definition 2.5: Start signal sequence
A sequence of signals S0, S1, S2, S3, ..., Sn is called a start signal sequence of length n, if So is the zero signal of sufficient length and if each signal Sk of the signal sequence ends exactly at the time when the signal Sk+1 begins. The temporal length of the zero signal is sufficient if it is at least as long as the time required for a cortex signal to reach the basal ganglia via the activity neuron of its cortex cluster and to be converted there into a climbing fiber signal in order to reach the golgi cells in the associated cerebellum cluster via the climbing fiber distribution neurons (striosome delay).
Each start signal sequence thus begins with a zero signal of sufficient length of about 125 milliseconds. A cortex signal needs this estimated time to reach the cerebellum as a climbing fiber signal. If the speed of the signals on the parallel fibres is about ten times lower than the speed on the moss fibres, the Purkinje cells would receive a start signal of 1/8 second length for a total of 9/8 seconds before the incoming climbing fibre signal interrupts the further signal supply at the Golgi cell. During the first eighth of a second, the signal rises from the moss fibres via the granule cells to the parallel fibres. Then the climbing fibre signal arrives and inhibits the relevant golgi cells with the exception of the first free Purkinj group. As the parallel fibres have already received the signal for the period of 1/8 second, the signal propagates at a tenfold reduced speed on the parallel fibres. Therefore the free Purkinje group receives this signal as a sequence of echoes. The first echo comes from the neighbouring Purkinje group. The second echo comes from the neighboring group of this neighboring group.
If the start signal S1 had a duration of ¼ second and if a successor signal S2 with a duration of one second would follow exactly after it, both of which may still belong to the signals which have not yet been coined, the excitation of signal S1 would continue to have an effect on the parallel fibres of the next free Pukinj group for one second after its termination, while signal S2 would also have an excitation effect for one second. In addition, the climbing fiber signal would be active, because both signals should be unexcited. Therefore they would now be imprinted in the next free Purkinje group. The signal of this Purkinje group would be the binary union of signal S1 and signal S2. This is because both the parallel fibers to S1 were active during the embossing process (due to the time stretching along the secondary parallel fibers), as well as the parallel fibers of signal S2.
As a result, complex signals are now generated which represent a stage two start signal sequence.
An analogy would be the formation of syllables of length two. A starting letter, immediately followed by a following letter, forms a syllable of two letters. The Cerebellum can now store exactly such signals in a single Purkinje cell. The special feature is that there are now signal predecessors and signal successors, whereby their temporal arrangement is of great importance. The signal "am" is different from the signal "ma" and is stored in a different Purkinje cell and is recognized there.
However, because the output of the cerebellum nuclei is (usually) fed to the thalamus as an excitatory signal, which in turn projects the thalamus into the cortex, the cortex in turn sends its signals via the bridge nuclei into the moss fibre system of the cerebellum, where the magnocellular climbing fibre signals also arrive, the process of storing start signal sequences is recursive.
In the first stage, start signal sequences of stage two are formed and assigned to new complex signals in the cerebellum. These end up in the cortex again with the remaining learned complex signals of the cerebellum. From there the projection into the cerebellum takes place again. Now new sequences of start signals can be formed. Everything is repeated as often as there are projections from the cerebellum to the thalamus and projections from the cortex to the cerebellum.
Theorem 2.16: Storage of start signal sequences in the cerebellum
The Cerebellum can learn start signal sequences of increasing length in a recursive algorithm. This is based on the time delay of the magnocellular climbing fiber projection of start signals into the cerebellum and the lower propagation speed of the signals along the parallel fibers. The signal of a start component is still effective after the end of the signal and can be bound to a new start signal sequence with the following signal that has arrived in the meantime.
This shows how a signal enters into a bond with its immediate successor, resulting in a complex signal which is output by the cerebellum in response to the start signal sequence. Since this process is recursive, start signal sequences of any length are created.
This would clarify in principle how the cerebellum can store a poem in a single Purkinje cell, for example. At first it forms "double letters", i.e. syllables with two partners. Then it forms syllables with three letters from letters and "double letters". At some point it has learned all syllables that are possible in the German language, for example. Each of them is stored in a separate Purkinje cell. Later, word sequences are stored in their own Purkinje cells, and from a certain level on, even a longer poem fits into a single Purkinje cell.
This is a compression method which leads to a never before known release of cerebellar memory. Therefore, from a systems-theoretical point of view, the emergence of language is primarily a compression method to gain storage space that would have been wasted if the language had been stored sequentially letter by letter. The freed memory was used to store the knowledge that mankind has acquired in the course of its evolutionary development. As a communicative tool for this transfer of knowledge, language was again used as a tool. Without it, every human being would have to make every experience himself, for which the available lifetime would not even be enough. In this respect, language also represents lived life.
What is not answered here is the question whether the moss fibres also divide into different sub-axons, so that there would not be only one cerebellum cluster per cortex cluster.
In this monograph only the magnocellular and parvocellular cerebellar systems were presented. The circuitry of the cerebellum, which uses the climbing fibers of the receptor system, cannot be shown here. Here the reader is put off until later. Also the use of the climbing fibers, which are derived from the cerebellum output itself, is not presented in this paper. The associated circuitry, which serves to "bind" complex signals to new complex signals, will (perhaps) be presented in the next monograph.
The binding of a signal sequence to a complex signal presented here requires a previous signal pause. In part 2.12 an algorithm is presented, which combines signal sequences to complex signals, but does not need this restrictive condition of a preceding signal pause.
ISBN
978-3-00-037458-6
ISBN 978-3-00-042153-2
Monografie von Dr. rer. nat. Andreas Heinrich Malczan